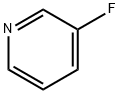
3-Fluoropyridine synthesis
- Product Name:3-Fluoropyridine
- CAS Number:372-47-4
- Molecular formula:C5H4FN
- Molecular Weight:97.09
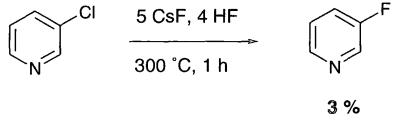

2875-18-5
266 suppliers
$12.00/1g

372-47-4
366 suppliers
$5.00/1g
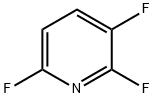
3512-18-3
114 suppliers
$34.00/1g
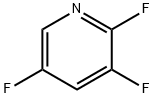
76469-41-5
132 suppliers
$14.00/1g
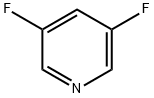
71902-33-5
164 suppliers
$6.00/1g
Yield:76469-41-5 24 %Spectr. ,3512-18-3 7 %Spectr. ,71902-33-5 15 %Spectr. ,372-47-4 8 %Spectr.
Reaction Conditions:
with triethylsilane;[Rh(μ-H)(1,3-bis(diisopropylphosphanyl)propane)]2 in benzene-d6 at 50; for 48 h;Inert atmosphere;regioselective reaction;
Steps:
4.11 Catalytic Hydrodefluorination of fluoroarenes with [Rh(μ-H)(dippp)]2 (1) as catalytic precursor
General procedure: To a solution of fluoroarene (0.1 M) and HSiEt3 (0.1 M) in benzene-d6 in a PFA tube α,α,α-trifluorotoluene (1-2 μL) was added as internal standard. The PFA tube was closed by a Teflon plug, inserted into an NMR tube and an initial 19F{1H} NMR spectrum was recorded. Then [Rh(μ-H)(dippp)]2 (1) (0.005 M) was added and the reaction mixture was heated to 50 °C for 48 h. Hydrodefluorination of pentafluoropyridine gave 2,3,5,6-tetrafluoropyridine (11%), 2,3,4,5-tetrafluoropyridine (11%), 2,3,5-trifluoropyridine (8%), 3,5-difluoropyridine (6%) and 2-fluoropyridine (1%) (TON = 11). Hydrodefluorination of 2,3,5,6-tetrafluoropyridine or 2,3,5,6-tetrafluoropyridine or 2,3,5,6-tetrafluoropyri-dine gave 2,3,5-trifluoropyridine (24%), 2,3,6-trifluoropyridine (7%), 3,5-difluoropyridine (15%), 2,5-difluoropyridine (2%) and 2-fluoropyridine (8%) (TON = 18). Hydrodefluorination of hexafluoro-benzene or hexafluoroben-zene or hexa-fluorobenzene gave pentafluorobenzene (12%) and 1,2,4,5-tetra-fluorobenzene or 1,2,4,5-tetrafluoro-benzene or 1,2,4,5-tetrafluoroben-zene (2%) (TON = 3.1). Hydrodefluorination of pentafluorobenzene gave 1,2,4,5-tetrafluorobenzene (35%), 1,2,3,4-tetrafluorobenzene (3%), 1,2,4-trifluorobenzene (23%) and 1,4-difluorobenzene (4%) (TON = 19). Yields of organic hydrodefluorination products were determined from 19F{1H} NMR spectra by integration of product resonances versus the internal standard. Hydrodefluorination products were identified by NMR spectroscopy by comparison with literature data [23]. TON: number of hydrodefluorination steps/moles of 1.
References:
Zámostná, Lada;Ahrens, Mike;Braun, Thomas [Journal of Fluorine Chemistry,2013,vol. 155,p. 132 - 142]
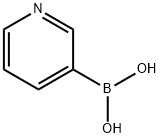
1692-25-7
476 suppliers
$5.00/1g

372-47-4
366 suppliers
$5.00/1g
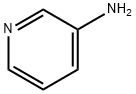
462-08-8
469 suppliers
$14.00/25g

372-47-4
366 suppliers
$5.00/1g
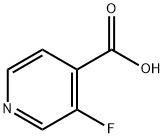
393-53-3
195 suppliers
$10.00/1g

372-47-4
366 suppliers
$5.00/1g
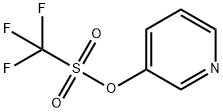
107658-27-5
40 suppliers
$126.00/5g

372-47-4
366 suppliers
$5.00/1g