
1-(4-amino-3-methoxyphenyl)piperidin-4-ol synthesis
- Product Name:1-(4-amino-3-methoxyphenyl)piperidin-4-ol
- CAS Number:761440-87-3
- Molecular formula:C12H18N2O2
- Molecular Weight:222.28

761440-22-6
18 suppliers
inquiry

761440-87-3
24 suppliers
inquiry
Yield:761440-87-3 411 mg
Reaction Conditions:
Stage #1: 1-(3-methoxy-4-nitrophenyl)piperidin-4-olwith ammonium chloride in tetrahydrofuran;methanol; for 0.166667 h;
Stage #2: with zinc in tetrahydrofuran;methanol at 20; for 1 h;
Steps:
13.2 Step 2 Preparation of compound 4A-6
The compound 3A-6 (0.58 g, 2.28 mmol) was dissolved in an aqueous solution of THF/MeOH (v/v=1:1, 20 mL in total) and saturated ammonium chloride (10 mL), with stirring for 10 min. Zinc powder (1.6 g) was added in portions, and the mixture was stirred at room temperature for 1 hour. After TLC indicated the reaction was completed, the reaction mixture was filtered and concentrated under reduced pressure to give a crude product, which was further isolated by column chromatography to obtain a compound 4A-6 (411 mg, yield 80.4%).
References:
EP3372594,2018,A1 Location in patent:Paragraph 0134; 0136
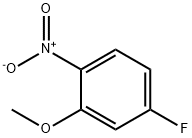
448-19-1
296 suppliers
$5.00/1g

761440-87-3
24 suppliers
inquiry
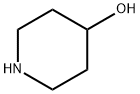
5382-16-1
13 suppliers
$9.00/5G

761440-87-3
24 suppliers
inquiry