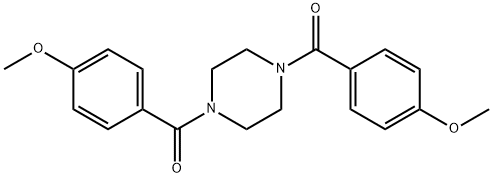
1,4-bis(4-methoxybenzoyl)piperazine synthesis
- Product Name:1,4-bis(4-methoxybenzoyl)piperazine
- CAS Number:116435-87-1
- Molecular formula:C20H22N2O4
- Molecular Weight:354.4
Yield:116435-87-1 86%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20;
Steps:
Compounds 25 and 26 were prepared as the following procedure
General procedure: The substituted acetyl chloride and piperazine were dissolved in DMF and K2CO3 with 2 to 1mol ratio, and the mixture was stirred at room temperature until the reaction was completed. The reaction was quenched with Na2CO3 aqueous solution and stirred overnight, and the precipitated product was collected via filtration and washed with water and dried to give the corresponding compounds. Piperazine-1,4-diylbis((4-methoxyphenyl)methanone) (25) White solid, melting point 215°C decomposed, 86% yield for the last step; 1H-NMR (400MHz, DMSO-d6) δ 7.403 (4H, d, J= 8.4Hz), 6.994 (4H, d, J= 8.4Hz), 3.799 (6H, s), 3.542 (8H, br). 13C-NMR (100MHz, DMSO-d6) δ 169.643, 160.793, 129.640, 127.996, 114.152, 55.746. DUIS-MS calculated for C20H22N2O4 [M+H]+: 355.1, found: 354.4.
References:
Petty, Aaron;Idippily, Nethrie;Bobba, Viharika;Geldenhuys, Werner J.;Zhong, Bo;Su, Bin;Wang, Bingcheng [European Journal of Medicinal Chemistry,2018,vol. 143,p. 1261 - 1276]
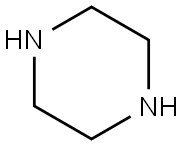
110-85-0
588 suppliers
$5.00/5G
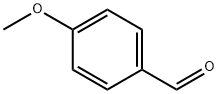
100-07-2
384 suppliers
$35.00/25g

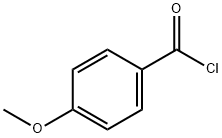
116435-87-1
12 suppliers
inquiry
94747-49-6
52 suppliers
$19.00/1g