
1-(4-bromo-2-fluorophenyl)ethanol synthesis
- Product Name:1-(4-bromo-2-fluorophenyl)ethanol
- CAS Number:353282-88-9
- Molecular formula:C8H8BrFO
- Molecular Weight:219.05
Yield:353282-88-9 94%
Reaction Conditions:
Stage #1: methyl iodidewith magnesium in diethyl ether;
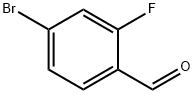
Stage #2: 4-bromo-2-fluorobenzaldehyde in diethyl ether at 20; for 12 h;Cooling with ice;
Steps:
19.1
A solution of 4-bromo-2- fluorobenzaldehyde (5 g, 24.62 mmol) in diethyl ether (10 ml) was added to an ice-cold solution of methylmagnesium iodide prepared from magnesium (1.7 g, 73.88 mmol) and methyliodide (4.58 ml, 73.88 mmol) in diethyl ether (50 ml). The mixture was warmed stirred at RT. for 12h, and cooled to 0°C, quenched with dil. 6N HC1 and extracted with ethyl acetate. The organic layer was dried over sodium sulphate and concentrated under reduced pressure to afford the title compound as red colour liquid (5 g, 94%). ^-NMR (δ ppm, CDC13, 400 MHz): δ 7.40 (t, J = 8.2 Hz, 1H), 7.30 (dd, J = 8.3, 1.7 Hz, 1H), 7.21 (dd, J = 9.9, 1.9 Hz, 1H), 5.17 (q, J = 6.4 Hz, 1H), 1.49 (d, J = 6.5 Hz, 3H).
References:
WO2011/145035,2011,A1 Location in patent:Page/Page column 114

75-16-1
265 suppliers
$12.00/10ml
57848-46-1
386 suppliers
$9.00/1g

353282-88-9
16 suppliers
inquiry
917-64-6
146 suppliers
$45.00/10ml

57848-46-1
386 suppliers
$9.00/1g

353282-88-9
16 suppliers
inquiry
676-58-4
277 suppliers
$15.00/25ml

57848-46-1
386 suppliers
$9.00/1g

353282-88-9
16 suppliers
inquiry
