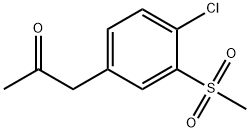
1-(4-chloro-3-(Methylsulfonyl)phenyl)propan-2-one synthesis
- Product Name:1-(4-chloro-3-(Methylsulfonyl)phenyl)propan-2-one
- CAS Number:593960-75-9
- Molecular formula:C10H11ClO3S
- Molecular Weight:246.71

74-88-4
343 suppliers
$15.00/10g

593960-75-9
8 suppliers
inquiry
Yield:593960-75-9 67%
Reaction Conditions:
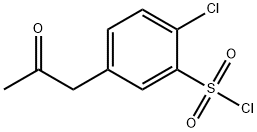
Stage #1: 2-chloro-5-(2-oxopropyl)-benzenesulphonyl-chloridewith sodium hydrogencarbonate;sodium sulfate in water at 70; for 1 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 40; for 1 h;
Steps:
5.2
A mixture of Na2SO3 (3.79g, 0.030mol) and NaHCO3 (2.53g, 0.030mol) in water (90ml) was stirred at 7O0C. To this solution was added a solution of Stage 1 product (4.65g, 0.015mol) in dioxane (190ml). Stirring was continued at 7O0C for 1 h. Upon cooling to r. t. the reaction mixture was concentrated to dryness in vacuo giving a brown solid. DMF (190ml) was added followed by MeI (1.88ml, 0.030mol). The reaction mixture was stirred at 400C for 1 h. After completion the reaction mixture was poured into water (90ml) and extracted with EtOAc (500ml). The EtOAc was dried (Na2SO4), filtered and concentrated in vacuo to give the title compound as a brown solid (2.49g, 67%) which was used in the next step without further purification. LCMS purity 81%, m/z 247 [M++H]; 1H NMR (400 MHz, CDCI3), δ: 2.15 (3H, s), 3.20 (3H, s), 3.75 (2H, s), 7.35 (1 H, d), 7.45 (1 H, d), 7.85 (1 H, s).
References:
WO2006/117567,2006,A2 Location in patent:Page/Page column 83