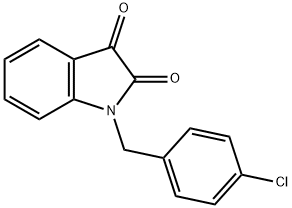
1-(4-CHLORO-BENZYL)-1H-INDOLE-2,3-DIONE synthesis
- Product Name:1-(4-CHLORO-BENZYL)-1H-INDOLE-2,3-DIONE
- CAS Number:26960-66-7
- Molecular formula:C15H10ClNO2
- Molecular Weight:271.7
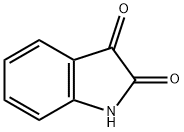
91-56-5
468 suppliers
$5.00/25g
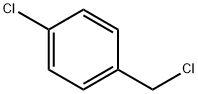
104-83-6
384 suppliers
$15.00/50g

26960-66-7
21 suppliers
$57.50/250mg
Yield:26960-66-7 84%
Reaction Conditions:
Stage #1: indole-2,3-dionewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.5 h;
Stage #2: 1-Chloro-4-(chloromethyl)benzene in N,N-dimethyl-formamide;mineral oil at 20; for 12 h;
Steps:
4.1.1. General procedure for synthesis of (E)-2-(1-(4-chlorobenzyl)-2-oxoindolin-3-ylidene) -N-(pyridin-4-yl)acetamide (4)
General procedure: To a solution of isatin (300 mg, 2.04 mmol) in DMF (10 mL) was added NaH (60% dispersion in mineral oil, 86 mg, 2.14 mmol) portionwise at 0 °C. The mixture was stirred at 0 °C for 30 min and 4-chlorobenzyl chloride (328 mg, 2.04 mmol) was added dropwise.The reaction mixture was stirred at room temperature for 12 h until complete by TLC. The reaction was quenched by addition of water and extracted with ethyl acetate. The combined organic layers were washed with brine solution, dried over MgSO4 and concentrated in vacuo. The residue was purified by flash column chromatography furnished the desired N-4-chlorobenzyl isatin (466 mg, 84%) as anorganic solid.
References:
Chiou, Chun-Tang;Lee, Wei-Chun;Liao, Jiahn-Haur;Cheng, Jing-Jy;Lin, Lie-Chwen;Chen, Chih-Yu;Song, Jen-Shin;Wu, Ming-Hsien;Shia, Kak-Shan;Li, Wen-Tai [European Journal of Medicinal Chemistry,2015,vol. 98,p. 1 - 12]
106-43-4
352 suppliers
$14.00/5g

91-56-5
468 suppliers
$5.00/25g

26960-66-7
21 suppliers
$57.50/250mg

91-56-5
468 suppliers
$5.00/25g
622-95-7
232 suppliers
$6.00/1g

26960-66-7
21 suppliers
$57.50/250mg