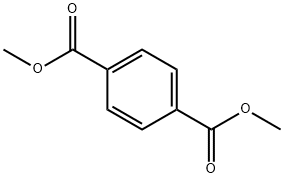
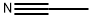1,4-DI(CYANOACETYL)BENZENE synthesis
- Product Name:1,4-DI(CYANOACETYL)BENZENE
- CAS Number:4640-70-4
- Molecular formula:C12H8N2O2
- Molecular Weight:212.2
Yield:4640-70-4 75%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;N,N-dimethyl-formamide at 100; for 2 h;
Steps:
6.1.2. Preparation of 3-oxopropanenitrile derivatives 2a, b
General procedure: 6.1.2.1. General procedure. A three-necked, round bottomed flask(500-ml), equipped with a magnetic stirrer, thermometer, decanter and condenser was charged with the proper ester 1a or 1b (10g),acetonitrile (10 ml, 10 mmol), in dry THF and stirred well, then an equivalent weight of NaH and DMF were added then refluxed to 100 °C for 2 h then left to cool, and filtered washed with petroleum ether. The formed salt was dissolve in ice-cold water and acidified HCl to afford the corresponding 3-oxo-propanenitrile derivatives 2a and 2b; respectively. 3,3'-(1,4-Phenylene)bis(3-oxopropanenitrile) (2a) [33] palebrown powder (EtOH/DMF), m.p:175-177 °C, yield:75% C12H8N2O2,IR (KBr, cm1): n 2549 (CΞN), 1717 (C]O); 1HNMR (DMSO-d6)3.875 (s, 4H, H2C), 8.03-8.07 (s, 4H, H-Ar), 13C NMR (DMSO-d6):29.3 (CH2), 129.9 (CH), 134.4(CΞN), 135.4 (CH), 167.10 (C]O); MS,(m/z): 149 (M, 100.0%),163(38.46%), 212 (25.23%), 104(60.52%);Anal calcd C12H8N2O2 (212.20); C, 67.92; H, 3.80; N, 13.20%; Found:C, 67.88; H, 3.77; N, 13.24%.
References:
Farag, Ahmad M.;Fahim, Asmaa M. [Journal of Molecular Structure,2019,vol. 1179,p. 304 - 314]