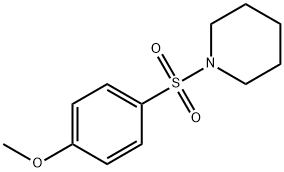
1-(4-Methoxyphenylsulfonyl)piperidine, 97% synthesis
- Product Name:1-(4-Methoxyphenylsulfonyl)piperidine, 97%
- CAS Number:35088-89-2
- Molecular formula:C12H17NO3S
- Molecular Weight:255.33
Yield:35088-89-2 96%
Reaction Conditions:
with tert.-butylhydroperoxide;ammonium iodide in tetrahydrofuran at 120; for 10 h;Sealed tube;
Steps:
8 Example 8: Synthesis of 1-((4-methoxyphenyl)sulfonyl)piperidine
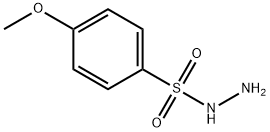
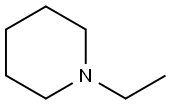
The substrate 60.6 mg (0.3 mmol) of 4-methoxybenzenesulfonylhydrazine and 51.0 mg (0.6 mmol) of piperidine, catalyst 4.3 mg (0.03 mmol) of ammonium iodide, 0.12 mL of t-butyl hydroperoxide and 2 mL of THF Add to a 30 mL sealed tube under air. The tube was then placed in a 120 ° C oil bath for 10 h. After completion of the reaction, the reaction mixture was cooled to room temperature, then 40 mL of brine, The organic layer was dried over anhydrous sodium sulfate, filtered, and evaporated, evaporated, .The obtained solid was subjected to hydrogen spectrum H NMR (400 MHz, CDCl3) δ 7.68 (d, J = 8.0 Hz, 2H), 7.01 (d, J = 8.0 Hz, 2H), 3.87 (s, 3H), 2.95. (t, J = 4.0 Hz, 4H), 1.63 (t, J = 4.0 Hz, 4H), 1.42 (d, J = 4.0 Hz, 2H), carbon spectrum C NMR (101 MHz, CDCl3) δ 162.80, 129.63 , 127.64, 114.05, 55.56, 46.87, 25.06, 23.40
References:
CN109020844,2018,A Location in patent:Paragraph 0034-0036

110-89-4
3 suppliers
$15.96/100ML

663175-94-8
13 suppliers
$215.00/500mg

35088-89-2
3 suppliers
inquiry