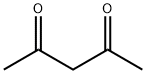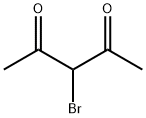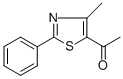
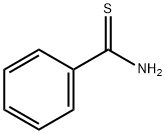
1-(4-METHYL-2-PHENYL-1,3-THIAZOL-5-YL)ETHAN-1-ONE synthesis
- Product Name:1-(4-METHYL-2-PHENYL-1,3-THIAZOL-5-YL)ETHAN-1-ONE
- CAS Number:7520-94-7
- Molecular formula:C12H11NOS
- Molecular Weight:217.29
Yield:7520-94-7 90%
Reaction Conditions:
in ethanol; for 8 h;
Steps:
4.1.1. General synthesis of 1-(4-methyl-2-(40-substitutedphenylthiazole-5-yl)ethane-1-one derivatives (methodA)
General procedure: According to the Hantzsch method, 3-chloro-2,4-pentandione isadded to the hot solution of aryl thioamide in absolute ethanol(Scheme 1) and boiled for 8 h. After being controlled with TLCwhere the reaction is completed, itwas left to cool, poured into coldwater and neutralized by sodium acetate solution. After the precipiationwas completed, it is taken and dried, crystallized fromethanol [40].4.1.1.1. 1-(4-Methyl-2-phenylthiazole-5-yl)ethane-1-one.Obtained according to Method A by the reaction of 3-chloro-2,4-pentanedione (110 mmol, 14.80 g) and thiobenzamide (100 mmol,13.72 g). Yield: %90, m.p.: 68-69 °C, m.p. reference: 68e70 C(Ethanol) [41]
References:
Sahin, Zafer;Biltekin, Sevde Nur;Yurttas, Leyla;Berk, Barkin;?zhan, Ya?mur;Sipahi, Hande;Gao, Zhan-Guo;Jacobson, Kenneth A.;Demirayak, ?eref [European Journal of Medicinal Chemistry,2021,vol. 212,art. no. 113125]
![Phosphonic acid, [1-hydroxy-1-(4-methyl-2-phenyl-5-thiazolyl)ethyl]-, diethyl ester (9CI)](/CAS/20210305/GIF/100288-98-0.gif)
100288-98-0
0 suppliers
inquiry

7520-94-7
15 suppliers
inquiry
![2,4-Pentanedione, 3-[[(4-methylphenyl)sulfonyl]oxy]-](/CAS/20180601/GIF/81447-35-0.gif)