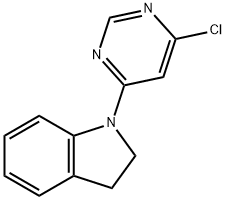
1-(6-Chloro-4-pyrimidinyl)indoline synthesis
- Product Name:1-(6-Chloro-4-pyrimidinyl)indoline
- CAS Number:293292-33-8
- Molecular formula:C12H10ClN3
- Molecular Weight:231.68
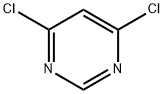
1193-21-1
564 suppliers
$14.00/5g
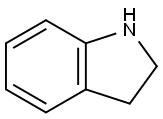
496-15-1
429 suppliers
$8.00/10g

293292-33-8
12 suppliers
$155.00/50mg
Yield:293292-33-8 89%
Reaction Conditions:
with triethylamine in ethanol at 20; for 17 h;
Steps:
13 General procedure for the preparation of chloroaminodiazines 3a-h, 5a-l, 7a, 7c, 9a-h
General procedure: To a solution of absolute ethanol (5 mL) and dichlorodiazine (3.36 mmol) in a 50mL round-bottom flaskwas added triethylamine (5.03 mmol), followed by the amine (5.03 mmol). The mixture was stirred either under reflux of ethanol (for dichloropyridazine and for dichloropyrazine) or at room temperature (for dichloropyrimidines). The reaction was monitored by GC. Once the starting dichlorodiazinewas completely consumed, the mixture was poured into a saturated NH4Cl solution (20 mL), then extracted with CH2Cl2 (320 mL). The combined organic layers were dried over Na2SO4, filtered, and evaporated under vacuum. The crude solid was triturated in petroleum ether, filtered through a Buchner to afford the pure product.
References:
Sengmany, Stéphane;Lebre, Julie;Le Gall, Ewan;Léonel, Eric [Tetrahedron,2015,vol. 71,# 29,p. 4859 - 4867]