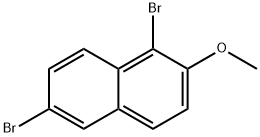
1,6-DIBROMO-2-METHOXYNAPHTHALENE synthesis
- Product Name:1,6-DIBROMO-2-METHOXYNAPHTHALENE
- CAS Number:66996-59-6
- Molecular formula:C11H8Br2O
- Molecular Weight:315.99
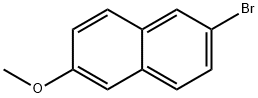
5111-65-9
336 suppliers
$6.00/5g

66996-59-6
30 suppliers
$45.00/100mg
Yield:66996-59-6 100%
Reaction Conditions:
with N-Bromosuccinimide in tetrahydrofuran; for 2 h;Reflux;
Steps:
14
6-Bromo-2-methoxynaphthalene (5.9 g, 24.81 mmol, 1 eq) and NBS (4.41, 24.81 mmol, 1 eq) are boiled in 50 ml of THF under reflux for 2 h. After washing with 1 N HCl, the organic phase is dried over magnesium sulfate, filtered and concentrated in vacuum on a rotary evaporator. The compound is obtained in quantitative yield (7.8 g). C11H8Br2O; MW 314/316/318; 1H-NMR (CDCl3): δ 7.98 (d, J=8.8 Hz, 1H), 7.84 (d, J=2.1 Hz, 1H), 7.63 (d, J=9.1 Hz, 1H), 7.50 (dd, J=2.1 Hz, J=9.1 Hz, 1H), 7.20 (d, J=9.1 Hz, 1H), 3.93 (s, 3H); 13C-NMR (CDCl3): δ 182.9, 157.9, 135.6, 134.8, 134.5, 133.7, 132.0, 131.9, 118.4, 60.9; IR: 2964, 1708, 1587, 1490, 1344, 1272, 1070 1/cm
References:
US2010/204234,2010,A1 Location in patent:Page/Page column 15
3401-47-6
165 suppliers
$59.00/5G

66996-59-6
30 suppliers
$45.00/100mg
16239-18-2
156 suppliers
$6.00/5g

74-88-4
340 suppliers
$15.00/10g

66996-59-6
30 suppliers
$45.00/100mg
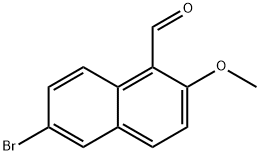
247174-18-1
17 suppliers
inquiry

66996-59-6
30 suppliers
$45.00/100mg
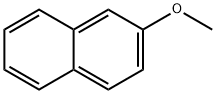
93-04-9
455 suppliers
$13.00/25g

66996-59-6
30 suppliers
$45.00/100mg