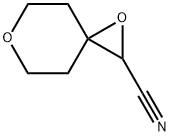
1,6-dioxaspiro[2.5]octane-2-carbonitrile synthesis
- Product Name:1,6-dioxaspiro[2.5]octane-2-carbonitrile
- CAS Number:883442-47-5
- Molecular formula:C7H9NO2
- Molecular Weight:139.15
29943-42-8
427 suppliers
$5.00/1g

107-14-2
282 suppliers
$15.00/25g
![1,6-dioxaspiro[2.5]octane-2-carbonitrile](/CAS/20180702/GIF/883442-47-5.gif)
883442-47-5
20 suppliers
inquiry
Yield:883442-47-5 83%
Reaction Conditions:
with potassium tert-butylate in tert-butyl alcohol;
Steps:
Intermediate 29 RRN 1701,6-Dioxaspiro[2.5]octane-2-carbonitrile
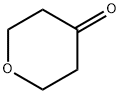
Intermediate 29 RRN 1701,6-Dioxaspiro[2.5]octane-2-carbonitrile [0380] dihydro-2H-pyran-4(3H)-one (2.5 g, 25 mmol) and 2-chloroacetonitrile (1.89 g, 25 mmol) was added a solution of potassium tert-butoxide in tert-butanol (1.0 M, 25 mL) dropwise. The reaction mixture was stirred overnight and quenched with water (50 mL). The mixture was extracted with ethyl acetate (100 mL). The organic layer was washed with water and brine, then dried over magnesium sulfate, filtered and concentrated. The crude product was purified via column chromatography (10-60% ethyl acetate/pet ether) to afford 1,6-dioxaspiro[2.5]octane-2-carbonitrile (2.9 g, 83% yield) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 3.95-3.80 (m, 4H), 3.35 (s, 1H), 2.18-2.01 (m, 1H), 1.96-1.76 (m, 2H), 1.67-1.50 (m, 1H).
References:
US2015/191464,2015,A1 Location in patent:Paragraph 0381