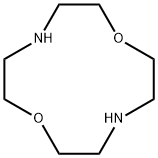
1,7-DIAZA-12-CROWN-4 synthesis
- Product Name:1,7-DIAZA-12-CROWN-4
- CAS Number:294-92-8
- Molecular formula:C8H18N2O2
- Molecular Weight:174.24
929-06-6
270 suppliers
$10.00/1g

110-91-8
678 suppliers
$9.00/1g

2752-17-2
161 suppliers
$65.00/1 G

294-92-8
60 suppliers
$45.00/1mg
Yield:-
Reaction Conditions:
with ammonia;chlorocarbonylhydrido[4,5-bis(dicyclohexylphosphinomethyl)acridine]ruthenium(II) in toluene at 155; under 31278.1 Torr; for 15 h;Autoclave;Inert atmosphere;
Steps:
3
Catalyst complex XIVb (for preparation, see below, weighed out under an inert atmosphere), solvent (such an amount that the total solvent volume is 50 ml) and the alcohol to be reacted were placed under an argon atmosphere in a 160 ml Parr autoclave (stainless steel V4A) having a magnetically coupled inclined blade stirrer (stirring speed: 200-500 revolutions/minute). The indicated amount of ammonia was introduced at room temperature either in precondensed form or directly from the pressurized NH3 gas bottle. If hydrogen was used, this was effected by iterative differential pressure metering. The steel autoclave was electrically heated to the temperature indicated and heated for the time indicated while stirring (500 revolutions/minute) (internal temperature measurement). After cooling to room temperature, venting the autoclave and outgassing the ammonia at atmospheric pressure, the reaction mixture was analyzed by GC (30m RTX5 amine 0.32 mm 1.5 μm). Purification of the particular products can, for example, be carried out by distillation. The results for the amination of 1,4-butanediol (table 1a, 1b), diethylene glycol (table 2) and monoethylene glycol (table 3), 2,5-furandimethanol (table 4), alkyldiols (table 5), 1,4-bis(hydroxymethyl)-cyclohexane (table 6) and aminoalcohols (table 7) are given below.
References:
BASF SE US2012/232293, 2012, A1 Location in patent:Page/Page column 12; 17
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

294-92-8
60 suppliers
$45.00/1mg
![4,10-bis[(4-methylphenyl)sulfonyl]-1,7-dioxa-4,10-diazacyclododecane](/CAS/20200119/GIF/52601-77-1.gif)
52601-77-1
1 suppliers
inquiry

294-92-8
60 suppliers
$45.00/1mg
![1,7-Dioxa-4,10-diazacyclododecane, 4-[(4-methylphenyl)sulfonyl]-](/CAS/20210305/GIF/96563-15-4.gif)
96563-15-4
0 suppliers
inquiry

294-92-8
60 suppliers
$45.00/1mg
![N,N-bis[2-(2-hydroxyethoxy)ethyl]-4-methylbenzenesulfonamide](/CAS/20180703/GIF/72358-83-9.gif)
72358-83-9
2 suppliers
inquiry

294-92-8
60 suppliers
$45.00/1mg