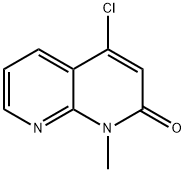
1,8-Naphthyridin-2(1H)-one, 4-chloro-1-methyl- synthesis
- Product Name:1,8-Naphthyridin-2(1H)-one, 4-chloro-1-methyl-
- CAS Number:1009360-86-4
- Molecular formula:C9H7ClN2O
- Molecular Weight:194.62
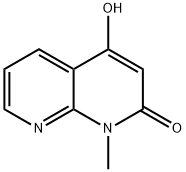
97510-72-0
3 suppliers
inquiry

1009360-86-4
3 suppliers
inquiry
Yield:1009360-86-4 24%
Reaction Conditions:
with trichlorophosphate at 100; for 2 h;
Steps:
4 preparation of 4-chloro-1-methyl-1,8-naphthyridin-2-one (compound L-5)
4-hydroxy-1-methyl-1,8-naphthyridin-2-one (compound L-4, 150.0 g, 850 mmol) in phosphorus oxychloride (300 mL) was stirred at 100 °C for 2 hours. The reaction mixture was concentrated in reduced pressure to remove the phosphorus oxychloride. The residue was neutralized by adding saturated aqueous NaHCO3 at room temperature to pH = 7 8, and the mixture was extracted with DCM (1000 mL) twice. The combined organic layer was washed with sat. brine (500 mL), dried over Na2SO4 and concentrated in vacuo to give a crude product, which was purified by silica gel chromatography (PE/EtOAc = 1:0 to 7:1) to afford compound L-5 (39 g, 24% yield). LCMS(M+H)+: 195, 1H NMR (400 MHz, DMSO-d6) ppm 8.75 (dd, J = 4.6, 1.6 Hz, 1H), 8.32 (dd, J = 7.9, 1.7 Hz, 1H), 7.44 (dd, J = 8.0, 4.6 Hz, 1H), 7.03 (s, 1H), 3.66 (s, 3H).
References:
WO2020/207991,2020,A1 Location in patent:Page/Page column 55-56
59514-93-1
33 suppliers
$109.00/100mg

74-88-4
340 suppliers
$15.00/10g

1009360-86-4
3 suppliers
inquiry

74-88-4
340 suppliers
$15.00/10g

1009360-86-4
3 suppliers
inquiry
2942-59-8
785 suppliers
$5.00/25g

1009360-86-4
3 suppliers
inquiry
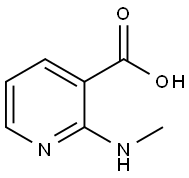
32399-13-6
61 suppliers
$60.00/100mg

1009360-86-4
3 suppliers
inquiry