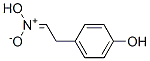
1-Aci-nitro-2-(p-hydroxyphenyl)ethane synthesis
- Product Name:1-Aci-nitro-2-(p-hydroxyphenyl)ethane
- CAS Number:37567-58-1
- Molecular formula:
- Molecular Weight:0

3179-08-6
68 suppliers
$6.00/1g

37567-58-1
3 suppliers
inquiry
Yield:37567-58-1 96%
Reaction Conditions:
with diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate;S-benzyl isothiouronium chloride in methanol at 60;Inert atmosphere;
Steps:
4.2. Typical procedure for the reduction of conjugated nitroalkenes
General procedure: The mixture of nitroalkenes 1 (0.1 g, 0.67 mmol), Hantzsch ester (0.19 g, 0.74 mmol) and S-benzyl isothiouronium chloride (14 mg, 0.067 mmol) in methanol (5 ml) was stirred at 60 °C for 5-12 h. After completion of the reaction, the crude product in methanol was concentrated and added into the excess cooled CH2Cl2. The S-benzyl isothiouronium chloride was readily precipitated out, and then filtered and washed many times with CH2Cl2 to be reused. The filtrate was evaporated and the residue was purified by flash column chromatography to give the required nitroalkanes products 2. All nitroalkanes except 2j and 2p were characterized with the reported spectroscopic data. [2] and [5]
References:
Nguyen, Quynh Pham Bao;Kim, Jae Nyoung;Kim, Taek Hyeon [Tetrahedron,2012,vol. 68,# 32,p. 6513 - 6516] Location in patent:supporting information; experimental part
22568-49-6
25 suppliers
$36.60/1g

37567-58-1
3 suppliers
inquiry
![Phenol, 4-[(1Z)-2-nitroethenyl]-](/CAS/20210305/GIF/1082703-37-4.gif)
1082703-37-4
0 suppliers
inquiry

37567-58-1
3 suppliers
inquiry

123-08-0
899 suppliers
$5.00/10g

37567-58-1
3 suppliers
inquiry
74213-97-1
0 suppliers
inquiry

37567-58-1
3 suppliers
inquiry