
4-HYDROXY-B-NITROSTYRENE synthesis
- Product Name:4-HYDROXY-B-NITROSTYRENE
- CAS Number:3179-08-6
- Molecular formula:C8H7NO3
- Molecular Weight:165.15
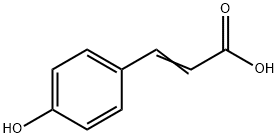
7400-08-0

3179-08-6
General procedure for the synthesis of 4-(2-nitrovinyl)phenol from 4-hydroxycinnamic acid: 0.01 mol of 4-hydroxycinnamic acid, about 0.01 mol of TCICA/DMF reagent, 1 g of sodium nitrite, and 25 mL of CH2Cl2 or acetonitrile were added to a pre-cleaned round-bottomed flask with stirring and the reaction was carried out under refluxing conditions for 8 to 10 hours. The progress of the reaction was monitored by TLC. Upon completion of the reaction, the reaction mixture was washed with NaHCO3 solution and subsequently extracted with ethyl acetate. The organic layer was separated, dried with anhydrous MgSO4 and concentrated in vacuum. Finally, purification by column chromatography using ethyl acetate-hexane as eluent gave the pure product 4-(2-nitrovinyl)phenol as a yellow powder.
Yield: 99%
Reaction Conditions:
AP-E1 at 90; for 0.25 - 0.266667 h;Product distribution / selectivity;Henry Reaction;
Steps:
Typically, 20 mg of the aminofunctionalized mesoporous sample was added into a solution of 122 mg (1 mmol) p-hydroxybenzaldehyde and 10 mL of nitromethane. The reaction was stirred at 90° C. (or at 50° C.) under nitrogen and aliquots of the reaction product were taken with a filter syringe and characterized by solution 1H NMR and GC-MS over the course of the reactions. The percent yield and conversion were determined by using 1H NMR (Bruker DPX-300 MHz) spectra. Resonances in acetone-d6were as follows:p-hydroxy nitrostyrene (1H NMR): δ 2.85 (1H, br, s), 6.96 (2H, d), 7.71 (2H, d), 7.83 (1H, d, J=13.5 Hz), and 8.04 (1H, d, J=13.5 Hz); andp-hydroxybenzaldehyde (1H NMR): δ 2.95 (1H, br, s), 7.05 (2H, d), 7.8 (2H, d), and 9.84 (1H, s).FIG. 44A depicts the catalysis time plots of AP-E1, AP-T1, AP-T2, AAP-E1, AAP-T1 and control sample MCM-41; and FIG. 44B depicts the catalysis time plots of sample AP-E1 compared with AP-EE1, AP-ET1, AP-EE2, and AP-ET2. Table 12 summarizes the applicants' mesoporous catalysts and control samples synthesized by grafting in various solvents and their catalytic efficiency in a reaction between p-hydroxybenzaldehyde and nitromethane. With regard to the data in Table 12, the Henry reaction was performed at 90° C. using nitromethane as the reactant and the solvent unless mentioned otherwise. The mmol NH2/g sample data were obtained from 29Si MAS NMR. TON is defined as the turnover number, i.e. mmol product/mmol catalyst. For samples AAP-E1 and AAP-T1, the reaction was done at 50° C. in nitromethane. For sample AP-E1, the reaction was performed at 90° C. using toluene as a solvent in the presence of a stoichiometric amount of nitromethane.The yields of the reaction were monitored over a course of reaction time by solution 1H NMR and GC-MS. For comparison purposes, the same amount of catalysts and reagents were used in the reactions. As can be seen in FIGS. 44A and 44B, both the monoamine- and diamine-functionalized samples synthesized in ethanol, AP-E1 and AAP-E1, were much more efficient than the corresponding samples grafted in toluene, AP-T1, AP-T2, and AP-T3, despite the former samples having about four times lower numbers of organoamine groups than the latter. The AP-E1 and AAP-E1 gave very nearly 100% yield in 15 and 30 minutes; corresponding turn-over-numbers (TON) of 37.5 and 7.6; and turn-over-frequencies of 150.0/h and 30.4/h, respectively. To the best of the applicants' knowledge, these values indicate these materials to be the most efficient catalysts of all mesoporous materials reported for Henry reactions in the literature to date.
References:
SYRACUSE UNIVERSITY US2009/43134, 2009, A1 Location in patent:Page/Page column 24

7400-08-0
459 suppliers
$29.00/10g

3179-08-6
74 suppliers
$6.00/1g

75-52-5
0 suppliers
$31.19/100g
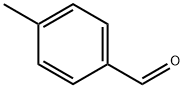
104-87-0
516 suppliers
$6.00/25g

123-08-0
961 suppliers
$5.00/10g
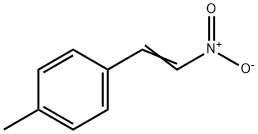
7559-36-6
27 suppliers
inquiry

3179-08-6
74 suppliers
$6.00/1g

75-52-5
0 suppliers
$31.19/100g

123-08-0
961 suppliers
$5.00/10g
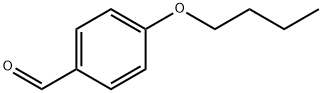
5736-88-9
155 suppliers
$10.00/1g

3179-08-6
74 suppliers
$6.00/1g
![1-butoxy-4-[(E)-2-nitroethenyl]benzene](/CAS/20200331/GIF/115514-08-4.gif)
115514-08-4
2 suppliers
inquiry

75-52-5
0 suppliers
$31.19/100g

123-08-0
961 suppliers
$5.00/10g
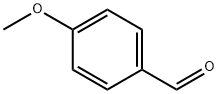
123-11-5
883 suppliers
$10.00/10g

3179-08-6
74 suppliers
$6.00/1g
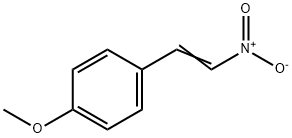
3179-10-0
51 suppliers
$44.00/1 g