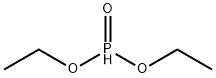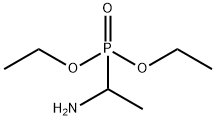
1-Aminoethylphosphonic acid diethyl synthesis
- Product Name:1-Aminoethylphosphonic acid diethyl
- CAS Number:54788-35-1
- Molecular formula:C6H16NO3P
- Molecular Weight:181.17
![Phosphonic acid, [1-[(trifluoroacetyl)amino]ethyl]-, diethyl ester (9CI)](/CAS/20210305/GIF/66788-53-2.gif)
66788-53-2
0 suppliers
inquiry

54788-35-1
6 suppliers
inquiry
Yield:54788-35-1 97%
Reaction Conditions:
with sodium tetrahydroborate in ethanol at 20; for 5 h;Reflux;
Steps:
Diethyl 1-aminoethylphosphonate or D/L-Fos diethyl ester (9)
Diethyl (1-(2,2,2-trifluoroacetamido)ethyl)phosphonate (8) (20.0 mmol, 5.6 g) was dissolved in ethanol (200 ml) andexcess sodium borohydride (200.0 mmol, 7.7 g) was added slowly with stirring. The resulting mixturewas stirred at room temperature for 1 hour, then heated at reflux for 4 hours. The mixture was cooledto room temperature and the solvent was removed in vacuo to afford a white solid, which wasdissolved in saturated NaHCO3 (96 g/L) (60 mL) with the addition of 10% aqueous K2CO3 (20 mL).The product was extracted into DCM (6 x 30 mL) and dried over MgSO4. The filtrate was concentratedin vacuo to afford a pale yellow liquid and purified by column chromatography using a gradientelution (DCM (100) to DCM/MeOH (90:10)) to afford 9 as a yellow liquid, a mixture of enantiomers(3.5 g, 19.3 mmol, 97%); max/cm-1 3431 (NH), 1215 (P = O), 1020 (P-O-C), 967 (P-O-C); 1H NMR (300MHz, CDCl3) δH 1.26 (6H, t, 3JH-H = 7.2 Hz, 2 x OCH2CH3), 1.34 (3H, dd, 3JH-P = 17.7 Hz, 3JH-H = 7.2 Hz,CH3-2), 1.68 (2H, br, NH2), 3.02-3.12 (1H, m, CH-1), 4.06-4.17 (4H, m, 2 x OCH2CH3); 13C NMR (75MHz, CDCl3) δC 16.4 (OCH2CH3), 16.5 (OCH2CH3), 17.2 (CH3-2), 44.2 (d, 1JC-P = 148.5 Hz, CH-1), 62.1(d, 2JC-P = 7.5 Hz, OCH2CH3), 62.1 (d, 2JC-P = 7.5 Hz, OCH2CH3); 31P-1Hdecoup NMR (121 MHz, CDCl3) δP29.6; HRMS (NSI) calcd for (C6H17NO3P)+, MH+: 204.0760, found 204.0762. LCMS purity >95% (C-18reversed phase, MeOH-H2O).
References:
Anderson, Rosaleen J.;Gray, Mark;Marrs, Emma C. L.;Ng, Keng Tiong;Orenga, Sylvain;Perry, John D. [Molecules,2020,vol. 25,# 7] Location in patent:supporting information
![Carbamic acid, N-[1-(diethoxyphosphinyl)ethyl]-, phenylmethyl ester](/CAS/20210305/GIF/54788-34-0.gif)
54788-34-0
0 suppliers
inquiry

54788-35-1
6 suppliers
inquiry

53145-08-7
0 suppliers
inquiry

54788-35-1
6 suppliers
inquiry

60593-26-2
0 suppliers
inquiry

54788-35-1
6 suppliers
inquiry