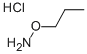
1-(AMMONIOOXY)PROPANE CHLORIDE synthesis
- Product Name:1-(AMMONIOOXY)PROPANE CHLORIDE
- CAS Number:6084-54-4
- Molecular formula:C3H10ClNO
- Molecular Weight:111.57
42832-43-9
0 suppliers
inquiry
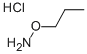
6084-54-4
28 suppliers
$65.00/50mg
Yield:6084-54-4 65.3%
Reaction Conditions:
with hydrogenchloride in ethanol;water at 65;
Steps:
4 Example 4 (E)-Glutazol-4-ene-3β-mercapto-n-propyl-6-one (Compound 7) and (E)-Glutosyl-3β,6β-mercapto-n-propyl-4-ene (compound 8)
Hydroxylamine hydrochloride (100.0 mg), ethyl acetate (240.0 μl),Sodium hydroxide (126.0 mg) was dissolved in 500 μl of deionized water, reacted under ice bath conditions, and monitored by TLC (thin silica gel plate) until the reaction was completed.After the reaction was completed, bromo-n-propane (191.6 μl) was added, and the reaction was carried out in an ice bath.TLC (thin layer silica gel plate) was monitored until the reaction was complete. After the reaction is completed,After extracting by-products with petroleum ether, the lower layer solution was taken and extracted three times with ethyl acetate.The ethyl acetate layer was taken, and the solvent was evaporated to dryness vacuo to yield white crystalFurther, ethanol (10.0 ml) and hydrochloric acid (15.0 μl) were added, and the reaction was carried out at 65 ° C.TLC (thin layer silica gel plate) was monitored until the reaction was complete. After the reaction is completed,The solvent was evaporated to give a pale yellow crystal of n-propyloxyamine hydrochloride (102.4 mg,The yield is 65.3%)
References:
CN106800580,2019,B Location in patent:Paragraph 0083-0085

51951-26-9
6 suppliers
inquiry
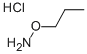
6084-54-4
28 suppliers
$65.00/50mg

5792-43-8
3 suppliers
inquiry
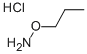
6084-54-4
28 suppliers
$65.00/50mg
64648-16-4
0 suppliers
inquiry
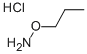
6084-54-4
28 suppliers
$65.00/50mg