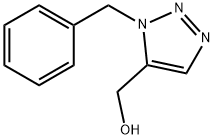
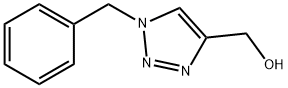
(1-Benzyl-1H-1,2,3-triazol-5-yl)Methanol synthesis
- Product Name:(1-Benzyl-1H-1,2,3-triazol-5-yl)Methanol
- CAS Number:77177-16-3
- Molecular formula:C10H11N3O
- Molecular Weight:189.21
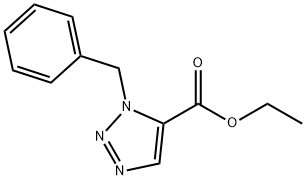
139029-46-2
0 suppliers
inquiry

77177-16-3
11 suppliers
inquiry
Yield:77177-16-3 99%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at -10 - 20; for 2 h;Inert atmosphere;
Steps:
V.b b. Preparation of intermediate 26
A solution of 1 .0M lithium aluminium hydride in tetrahydrofurane (8.30 mmol, 8.30 ml_, 4.0 eq.) was diluted in dry tetrahydrofurane (50 ml_) and cooled down to -10°C, under argon atmosphere. Compound 25 (480 mg, 2.07 mmol, 1 .0 eq.) was solubilized in dry tetrahydrofurane (50 ml_). This solution was added dropwise to the cooled solution of lithium aluminium hydride. Reaction mixture was allowed to warm up to RT and was stirred for 2h. After cooling down at 0°C a saturated solution of Na2S04 was carrefully added to the mixture. The resulting white precipitate/gum was removed by filtration over cotton pad, and the filtrate was extracted ethyl acetate (3 x 300 ml_), dried over magnesium sulfate and solvents were evaporated under vacuum, affording compound 26 (400 mg, 99% yield) as a brown oil.
References:
WO2019/243273,2019,A1 Location in patent:Page/Page column 41-42

107-19-7
5 suppliers
$12.00/25g
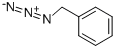
622-79-7
109 suppliers
$15.00/250mg
28798-81-4
37 suppliers
$49.47/250mgs:

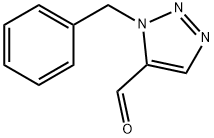
77177-16-3
11 suppliers
inquiry
139454-85-6
11 suppliers
inquiry

77177-16-3
11 suppliers
inquiry
![2-Propen-1-ol, 3-[(4-methylphenyl)sulfonyl]-](/CAS/20210305/GIF/41411-59-0.gif)
