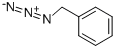
BENZYL AZIDE synthesis
- Product Name:BENZYL AZIDE
- CAS Number:622-79-7
- Molecular formula:C7H7N3
- Molecular Weight:133.15

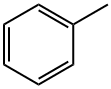
108-88-3
7 suppliers
$21.80/5 mL

160732-56-9
16 suppliers
inquiry
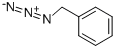
622-79-7
111 suppliers
$15.00/250mg
Yield:622-79-7 95.5%
Reaction Conditions:
with tris(2,2'-bipyridyl)ruthenium dichloride at 20; for 10 h;Irradiation;
Steps:
1 Example 1
In a 100 mL three-necked bottle, add 5 mL of toluene.2mol of azidoacetoxyiodobenzene 2,50mL hexafluoroisopropanol,0.02 mol of Ru(bpy)3Cl2,With a 10W blue light,Reaction at room temperature for 10 hours,Evaporate the solvent under reduced pressureThe residue is purified by column chromatography to give benzyl azide 3,Yield 95.5%.
References:
Yunnan Nationalities University;Gu Lijun;Li Ganpeng;Yang Guangyu;Zhang Hongtao CN107141235, 2017, A Location in patent:Paragraph 0030; 0031; 0032; 0033; 0034

100-46-9
462 suppliers
$5.00/5 g
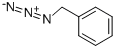
622-79-7
111 suppliers
$15.00/250mg
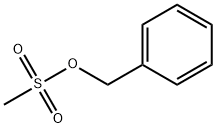
55791-06-5
24 suppliers
inquiry
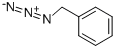
622-79-7
111 suppliers
$15.00/250mg

100-39-0
425 suppliers
$10.00/10 g
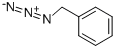
622-79-7
111 suppliers
$15.00/250mg

100-44-7
628 suppliers
$13.50/250G
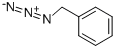
622-79-7
111 suppliers
$15.00/250mg