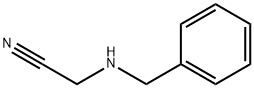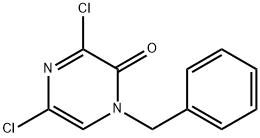
1-benzyl-3,5-dichloropyrazin-2(1H)-one synthesis
- Product Name:1-benzyl-3,5-dichloropyrazin-2(1H)-one
- CAS Number:87486-35-9
- Molecular formula:C11H8Cl2N2O
- Molecular Weight:255.1

79-37-8
465 suppliers
$17.67/10gm:

63086-36-2
20 suppliers
$44.89/5g

87486-35-9
17 suppliers
inquiry
Yield:87486-35-9 87%
Reaction Conditions:
Stage #1:oxalyl dichloride;2-(benzylamino)acetonitrile hydrochloride at 0 - 55; for 18 h;
Stage #2: with potassium dihydrogenphosphate in dichloromethane;water for 0.5 h;
Steps:
2.7 4.2.2.1 Synthesis of N-Alkylated 2-pyrazinones 4 and 5
General procedure: To a suspension of amino acetonitrile hydrochloride in toluene at 0 °C was added oxalyl chloride (5 equiv) and the reaction mixture was heated to 55 °C for 18 h. The resulting solution was concentrated in vacuo, the residue was dissolved in dichloromethane, treated with saturated KH2PO4 and stirred for 30 min. The organics were then extracted with dichloromethane, dried over MgSO4, filtered and concentrated in vacuo to yield the desired 2-pyrazinones, which were further purified by flash column chromatography on silica gel using ethyl acetate/petroleum ether 40-60° (20:80).
References:
Harker, Wesley R.R.;Delaney, Patrick M.;Simms, Michael;Tozer, Matthew J.;Harrity, Joseph P.A. [Tetrahedron,2013,vol. 69,# 5,p. 1546 - 1552]

100-46-9
461 suppliers
$5.00/5 g

87486-35-9
17 suppliers
inquiry