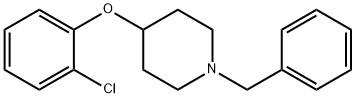
1-Benzyl-4-(2-chlorophenoxy)piperidine synthesis
- Product Name:1-Benzyl-4-(2-chlorophenoxy)piperidine
- CAS Number:900512-07-4
- Molecular formula:C18H20ClNO
- Molecular Weight:301.81
Yield:900512-07-4 89%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in dichloromethane at 0; for 72 h;
Steps:
43
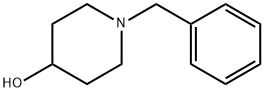
Preparation 43: 1-Benzyl-4-(2-chlorophenoxy)piperidine Triphenylphosphine (1.91 g, 7.31 mmol) was added to an ice-cooled solution of di-isopropylazodicarboxylate (1.48 g, 7.31 mmol) in dichloromethane (15 mL) and the mixture was stirred for 10 minutes. A solution of 2-chlorophenol (806 mg, 6.27 mmol) and 1-benzyl-4-hydroxypiperidine (1 g, 5.22 mmol) in dichloromethane (5 mL) was then added dropwise to the ice-cooled reaction mixture and stirring continued for a further 72 hours. The reaction mixture was then concentrated in vacuo and the residue was dissolved in diethyl ether and extracted with saturated citric acid solution (5×10 mL). The combined aqueous solution was then basified with sodium hydroxide and extracted with dichloromethane (2×20 mL). The combined organic solution was dried over magnesium sulfate and concentrated in vacuo to afford the title compound as a clear oil in 89% yield, 1.4 g. 1H NMR(CDCl3, 400 MHz) □: 1.85-1.95(m, 2H), 1.97-2.08(m, 2H), 2.32-2.47(m, 2H), 3.58(s, 2H), 4.46-4.34(m, 1H), 6.87-6.95(m, 2H), 7.16-7.22(m, 1H), 7.20(dd, 1H), 7.26-7.37(m, 6H)
References:
US2006/160786,2006,A1 Location in patent:Page/Page column 30