
1-benzyl-4-phenylpiperidine-4-methylamine synthesis
- Product Name:1-benzyl-4-phenylpiperidine-4-methylamine
- CAS Number:84176-77-2
- Molecular formula:C19H24N2
- Molecular Weight:280.41
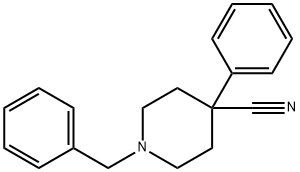
56243-25-5
90 suppliers
$37.39/5g

84176-77-2
21 suppliers
$35.00/200μmol
Yield:84176-77-2 92%
Reaction Conditions:
Stage #1: 1-benzyl-4-cyano-4-phenylpiperidinewith lithium aluminium tetrahydride in diethyl ether at 0 - 25;
Stage #2: with sodium hydrogencarbonate in diethyl ether;
Steps:
26.1
Step 1. l-[4-phenyl-l-(phenylmethyl)-4-piperidinyl]methanamine: A solution of 4-phenyl-l-(phenylmethyl)-4-piperidinecarbonitrile (4 g, 12.8 mmol) in dry Et2O (60 ml) at 0 0C was treated with LiAlH4 (0.585 g, 15.3 mmol) and the suspension was stirred at 25 0C overnight before being filtered through celite and concentrated under
References:
WO2008/124582,2008,A1 Location in patent:Page/Page column 131-132
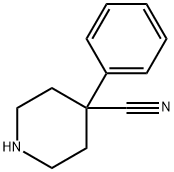
40481-13-8
59 suppliers
inquiry

84176-77-2
21 suppliers
$35.00/200μmol