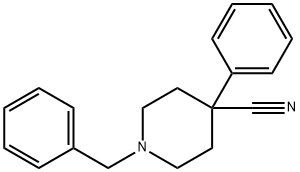
1-Benzyl-4-cyano-4-phenylpiperidine hydrochloride synthesis
- Product Name:1-Benzyl-4-cyano-4-phenylpiperidine hydrochloride
- CAS Number:56243-25-5
- Molecular formula:C19H20N2
- Molecular Weight:276.38
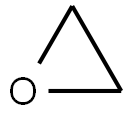
75-21-8
218 suppliers
$33.29/crm44609
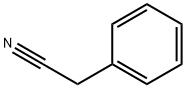
140-29-4
5 suppliers
$18.00/25mL

100-46-9
462 suppliers
$5.00/5 g

56243-25-5
90 suppliers
$37.39/5g
Yield: 54%
Reaction Conditions:
Stage #1:phenylacetonitrile with n-butyllithium;diisopropylamine in tetrahydrofuran at -30; for 0.5 h;Inert atmosphere;
Stage #2:oxirane in tetrahydrofuran for 0.5 h;Inert atmosphere;
Stage #3:benzylamineFurther stages;
Steps:
10 One-pot synthesis of 4-cyano-N-benzyl-4-phenylpiperidine:
Diisopropylamine (3.30mL, 24mmol) was dissolved in 20mL of anhydrous tetrahydrofuran, argon protectedThe temperature was lowered to -30 , 2.4mol / L n-butyllithium (10mL, 24mmol) was added dropwise, and the reaction was 0.5h; acetonitrile (1.17g, 10mmol) was added, and the reaction was continued to stir for 0.5h;Add ethylene oxide (1.06g, 24mmol) dropwise and react for 0.5h;Methanesulfonyl chloride (2.74g, 24mmol) was added dropwise, and the reaction was continued to stir for 0.5h;Slowly rise to 0 for 1h;Add triethylamine (5.05g, 50mmol), benzylamine (5.35g, 50mmol)The temperature was raised to 120 ° C and the reaction was closed in a steel reactor for 12h; the reaction was stopped and cooled to room temperature, tetrahydrofuran was distilled off, 50mL of dichloromethane was added and extracted with 20mL of water, the organic phase was separated, washed with saturated brine, and dried over anhydrous sodium sulfate. After filtration and concentration, a white solid was isolated by silica gel column chromatography with a total yield of 54%.
References:
The Chinese People's Liberation Army 63975 Unit;Gao Zhenhua;Zou Chuanpin;Zhong Hui;He Xiaowei;Liu Lihui;Meng Xiangyan;Jiao Jianlan CN103936665, 2016, B Location in patent:Paragraph 0149; 0153-0158
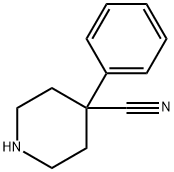
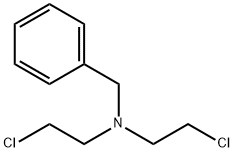
40481-13-8
59 suppliers
inquiry

100-39-0
423 suppliers
$10.00/10 g

56243-25-5
90 suppliers
$37.39/5g

100-39-0
423 suppliers
$10.00/10 g
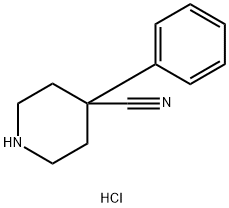
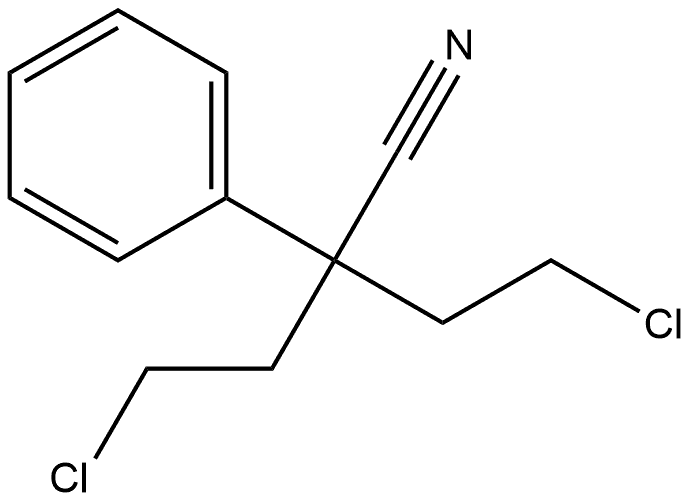
51304-58-6
78 suppliers
$13.43/1gm:

56243-25-5
90 suppliers
$37.39/5g