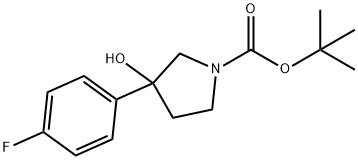
1-BOC-3-(4-FLUOROPHENYL)-3-HYDROXYPYRROLIDINE synthesis
- Product Name:1-BOC-3-(4-FLUOROPHENYL)-3-HYDROXYPYRROLIDINE
- CAS Number:1003560-58-4
- Molecular formula:C15H20FNO3
- Molecular Weight:281.32
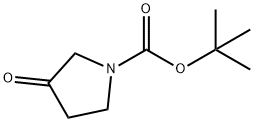
101385-93-7
473 suppliers
$5.00/1g
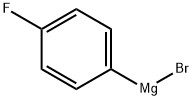
352-13-6
194 suppliers
$40.00/50mL

1003560-58-4
8 suppliers
inquiry
Yield:-
Reaction Conditions:
in tetrahydrofuran;diethyl ether at 20; for 0.583333 h;
Steps:
216
A solution of 4-fluorophenylmagnesium bromide (1M in THF, 11 mL, 11 mmol) was added gradually to a solution of tert-butyl 3-oxopyrrolidine-1-carboxylate (1.85 g, 10 mmol) in diethyl ether (40 mL) during 5 min at ambient temperature. The mixture was stirred for 30 min and thereafter slowly quenched with NH3Cl (10 mL, 3M). The organic phase was isolated and evaporated. The residue was purified by flash chromatography (SiO2 2:1 heptane/EtOAc) to afford the title compound, which was used without further purification. MS (EI) 208 (M-tBuO)
References:
US2008/21022,2008,A1 Location in patent:Page/Page column 96

101385-93-7
473 suppliers
$5.00/1g
460-00-4
614 suppliers
$6.00/10g

1003560-58-4
8 suppliers
inquiry