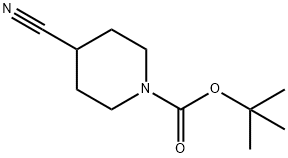
1-Boc-4-cyanopiperidine synthesis
- Product Name:1-Boc-4-cyanopiperidine
- CAS Number:91419-52-2
- Molecular formula:C11H18N2O2
- Molecular Weight:210.27
137076-22-3
348 suppliers
$5.00/1g

91419-52-2
363 suppliers
$6.00/1g
Yield:91419-52-2 73%
Reaction Conditions:
with potassium hexafluorophosphate;tert.-butylnitrite;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;oxygen;1,1,1,3,3,3-hexamethyl-disilazane in acetonitrile at 30; for 8 h;
Steps:
23 Example 23: Preparation of N-Boc-4-piperidinecarbonitrile (Formula (2-8))
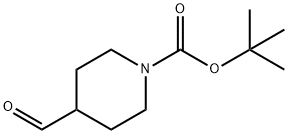
In a 100-ml flask, 30 mL of acetonitrile, 10 mmol of HMDS, 0.4 mmol of TEMPO, 0.4 mmol of KPF6 and 0.6 mmol of TBN were charged. The air was replaced with oxygen. The mixture was heated to 30 ° C in a preheated water bath and 4 mmol of N-Boc-4-piperidinecarboxaldehyde (as shown in formula (1-8)) was added slowly for 8 h. The reaction mixture was stirred with sodium thiosulfate solution and extracted with ether. The organic layer was separated, the solvent was distilled off under reduced pressure, and the residue was subjected to column chromatography. The mixture was stirred at a volume ratio of ethyl acetate / petroleum ether of 1: 100 The eluent containing the title compound was collected and the solvent was evaporated to give N-Boc-4-piperidinecarbonitrile in an isolation yield of 70%.; The reaction procedure was the same as in Example 20, except that the amount of acetonitrile used was 80 mL, and the isolated yield of N-Boc-4-piperidinecarbonitrile was 73%.
References:
Zhejiang University of Technology;Li, Meichao;Chen, Zhenlu;Tang, Chaojie;Mo, Weimin CN105481624, 2016, A Location in patent:Paragraph 0041; 0042
91419-48-6
220 suppliers
$20.80/1G

91419-52-2
363 suppliers
$6.00/1g
4395-98-6
230 suppliers
$14.00/1g

24424-99-5
869 suppliers
$13.50/25G

91419-52-2
363 suppliers
$6.00/1g
![1-PIPERIDINECARBOXYLIC ACID,4-[(HYDROXYIMINO)METHYL]-,1,1-DIMETHYLETHYL ESTER](/CAS/GIF/190446-85-6.gif)
190446-85-6
32 suppliers
$60.00/100mg

91419-52-2
363 suppliers
$6.00/1g
39546-32-2
344 suppliers
$6.00/5g

24424-99-5
869 suppliers
$13.50/25G

91419-52-2
363 suppliers
$6.00/1g