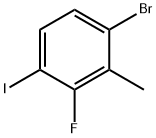
1-Bromo-3-fluoro-4-iodo-2-methyl-benzene synthesis
- Product Name:1-Bromo-3-fluoro-4-iodo-2-methyl-benzene
- CAS Number:1114546-29-0
- Molecular formula:C7H5BrFI
- Molecular Weight:314.92
105931-73-5
365 suppliers
$5.00/5g

74-88-4
351 suppliers
$15.00/10g

1114546-29-0
24 suppliers
inquiry
Yield:1114546-29-0 100%
Reaction Conditions:
Stage #1: with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 1 h;
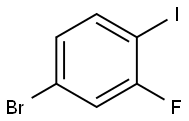
Stage #2: 1-bromo-3-fluoro-4-iodobenzene in tetrahydrofuran;hexane at -78; for 1 h;
Stage #3: methyl iodide in tetrahydrofuran;hexane;
Steps:
1 1-bromo-3-fluoro-4-iodo-2-methylbenzene
Reference Example 1 1-bromo-3-fluoro-4-iodo-2-methylbenzene A solution of diisopropylamine (6.06 mL) in THF (100 mL) was cooled to -78° C., n-butyllithium-hexane (24.9 mL, 1.6 mol/L) was added dropwise and, after the completion of the dropwise addition, the mixture was stirred at -78° C. for 1 hr. Subsequently, a solution of 4-bromo-2-fluoro-1-iodobenzene (10.0 g) in THF (50 mL) was added dropwise, and the mixture was further stirred at -78° C. for 1 hr. Methyl iodide (2.90 mL) was added dropwise at -78° C. and the mixture was further stirred at -78° C. for 2 hr, and the mixture was warmed to room temperature. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The extract was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to give the title compound as a brown oil (yield: 10.5 g, yield: 100%). 1H-NMR(CDCl3)δ:2.37(3H,d,J=2.6 Hz), 7.10(1H,dd,J=8.5,1.1 Hz), 7.39-7.46(1H,m).
References:
US2009/42967,2009,A1 Location in patent:Page/Page column 20-21