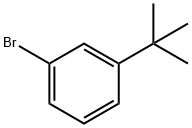
1-BROMO-3-TERT-BUTYLBENZENE synthesis
- Product Name:1-BROMO-3-TERT-BUTYLBENZENE
- CAS Number:3972-64-3
- Molecular formula:C10H13Br
- Molecular Weight:213.11
103273-01-4
139 suppliers
$8.00/1g

3972-64-3
149 suppliers
$9.00/1g
Yield:3972-64-3 84%
Reaction Conditions:
Stage #1: 2-bromo-4-tert-butylanilinewith sulfuric acid;acetic acid;sodium nitrite in water at 10;Cooling;
Stage #2: with ferrous(II) sulfate heptahydrate in water;N,N-dimethyl-formamide;
Steps:
1-Bromo-3-tert-butylbenzene (VII)
Synthesized according to a known hydrodediazoniation protocol.59Concd H2SO4 (10 mL, 188 mmol) was added to a solution of 2-bromo-4-tert-butylaniline (22.43 g, 98 mmol) in AcOH (160 mL). The solutionwas cooled in an ice-salt bath. A solution of NaNO2 (9 g, 130 mmol in40 mL of water) was added dropwise with vigorous stirring, the temperatureof the mixture was kept below 10 °C. The resulting solutionwas added to a mixture of FeSO4·7H2O (32 g, 115 mmol) and DMF(300 mL) with stirring. Afterwards, the mixture was diluted with water(1.25 L) and extracted with CH2Cl2. The combined organic layerswere washed with aq Na2CO3 and water, dried (Na2SO4), and concentratedunder reduced pressure. The residue was distilled under vacuumyielding 1-bromo-3-tert-butylbenzene (17.50 g, 84%); bp 78-83°C/2 Torr.1H NMR (400 MHz, CDCl3): = 7.51 (td, J = 1.9, 0.3 Hz, 1 H), 7.33-7.29(m, 2 H), 7.17 (ddd, J =8.1, 7.7, 0.3 Hz, 1 H), 1.31 (s, 9 H).
References:
Levitskiy, Oleg A.;Klimchuk, Ivan A.;Grishin, Yuri K.;Roznyatovsky, Vitaly A.;Tarasevich, Boris N.;Magdesieva, Tatiana V. [Synthesis,2022,vol. 54,# 6,p. 1601 - 1612]