
1-BROMO-5-FLUORO-2-METHOXY-3-NITROBENZENE synthesis
- Product Name:1-BROMO-5-FLUORO-2-METHOXY-3-NITROBENZENE
- CAS Number:179897-92-8
- Molecular formula:C7H5BrFNO3
- Molecular Weight:250.02
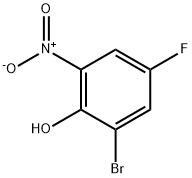
320-75-2
59 suppliers
$11.00/250mg

74-88-4
342 suppliers
$15.00/10g

179897-92-8
38 suppliers
$32.00/1g
Yield: 61.8%
Reaction Conditions:
with potassium carbonate in acetone at 80; for 22 h;
Steps:
2.2
Step 2 1-Bromo-5-fluoro-2-methoxy-3-nitro-benzene; 2-Bromo-4-fluoro-6-nitro-phenol 2b (24.7 g, 104.7 mmol) and potassium carbonate (17.34 g, 125.6 mmol) were dissolved in 300 mL of acetone followed by addition of iodomethane (9.8 mL, 157.1 mmol). The reaction mixture was heated to reflux at 80 °C for 22 hours. The reaction was monitored by TLC until the disappearance of the starting materials. The mixture was concentrated under reduced pressure and diluted with 200 mL of ethyl acetate and 200 mL of water. The aqueous layer was extracted with ethyl acetate (100 mL ×2). The combined organic extracts were washed with 4 N hydrochloric acid and saturated aqueous sodium bicarbonate and dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain the title compound 1-bromo-5-fluoro-2-methoxy-3-nitro-benzene 2c (16.18 g, yield 61.8%) as a white solid. MS m/z (ESI): 252 [M+1] 1H NMR (CDCl3): δ 3.99 (s, 3H), 7.81 (d, J = 8.0 Hz, 1H), 7.28 (q, J = 8.0 Hz, 4.0 Hz, 1H), 7.89 (q, J = 8.0 Hz, 4.0 Hz, 1H)
References:
Jiangsu Hengrui Medicine Co., Ltd.;Shanghai Hengrui Pharmaceutical Co. Ltd. EP2236500, 2010, A1 Location in patent:Page/Page column 17-18
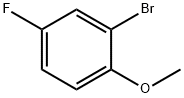
452-08-4
259 suppliers
$9.00/1g

179897-92-8
38 suppliers
$32.00/1g