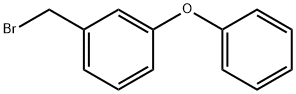
1-(BROMOMETHYL)-3-PHENOXYBENZENE synthesis
- Product Name:1-(BROMOMETHYL)-3-PHENOXYBENZENE
- CAS Number:51632-16-7
- Molecular formula:C13H11BrO
- Molecular Weight:263.13
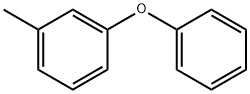
3586-14-9
195 suppliers
$9.00/5g

51632-16-7
94 suppliers
$74.23/250mgs:
Yield: 86%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane for 3 h;Reflux;
Steps:
5.2.13. 1-(Azidomethyl)-3-phenoxybenzene (13)
A solution of m-(phenoxy)toluene (3.68 g; 20 mmol) in 45 mL CCl4 was treated with N-bromosuccinimide (5.34 g; 30 mmol) and azo-bis(isobutyronitrile) (15 mg) and the reaction mixture was refluxed for 3 h. The solution was allowed to cool to room temperature and then placed in an ice bath. A white precipitate formed which was filtered off and the filtrate was evaporated, leaving pure compound 1 as a yellow oil (4.5 g; 86% yield). 1H NMR (CDCl3): δ 4.4 (s, 2H), 6.9-7.41 (m, 9H). This was used in the next step. To sodium azide (3.25 g; 50 mmol) in dry DMSO (80 mL) was added compound 12 (4.5 g; 17.1 mmol) and the reaction mixture was stirred overnight at room temperature. The reaction mixture was cooled in an ice bath and quenched with water (50 mL). The aqueous layer was extracted with ethyl ether (3 × 30 mL). The combined ethyl ether extracts were washed with water (2 × 20 mL) and dried over anhydrous sodium sulfate. The solvent was removed, leaving a crude product which was purified using flash chromatography (silica gel/methylene chloride/hexanes) to give compound 13 as a colorless oil (3.31 g; 60% yield). 1H NMR (CDCl3): δ 4.25 (s, 2H), 6.07-7.45 (m, 9H).
References:
Dou, Dengfeng;Mandadapu, Sivakoteswara Rao;Alliston, Kevin R.;Kim, Yunjeong;Chang, Kyeong-Ok;Groutas, William C. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 19,p. 5749 - 5755] Location in patent:experimental part
13826-35-2
311 suppliers
$6.00/5g

51632-16-7
94 suppliers
$74.23/250mgs:
558-13-4
252 suppliers
$10.00/1g

13826-35-2
311 suppliers
$6.00/5g

51632-16-7
94 suppliers
$74.23/250mgs:
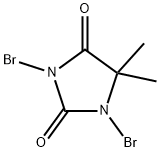
77-48-5
531 suppliers
$11.00/10g

3586-14-9
195 suppliers
$9.00/5g

51632-16-7
94 suppliers
$74.23/250mgs:
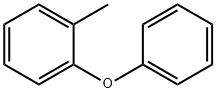
3991-61-5
1 suppliers
inquiry

3586-14-9
195 suppliers
$9.00/5g

51632-16-7
94 suppliers
$74.23/250mgs:

53874-67-2
1 suppliers
inquiry