
1-Cyclopropyl-4-iodopyridin-2(1H)-one synthesis
- Product Name:1-Cyclopropyl-4-iodopyridin-2(1H)-one
- CAS Number:1193335-00-0
- Molecular formula:C8H8INO
- Molecular Weight:261.0597

858839-90-4
89 suppliers
$52.00/250mg
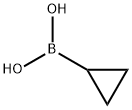
411235-57-9
426 suppliers
$6.00/1g

1193335-00-0
2 suppliers
inquiry
Yield:1193335-00-0 81%
Reaction Conditions:
with copper diacetate;sodium carbonate in 1,2-dichloro-ethane at 70; for 18 h;
Steps:
85.1
A mixture of 4-iodopyridin-2(1H)-one (0.2425 g, 1.10 mmol, 1.0 equiv), Cu(OAc)2 (0.2146 g, 1.18 mmol, 1.07 equiv), bipyridine (0.1832 g, 1.17 mmol, 1.07 equiv), cyclopropylboronic acid (0.2122 g, 2.47 mmol, 2.25 equiv) and Na2CO3 (0.2638 g, 2.49 mmol, 2.27 equiv) in dichloroethane (10 mL) was stirred at 70° C. for 18 h. The reaction mixture was quenched with satd aq NH4Cl, diluted with CH2Cl2, and dried over Na2SO4. After the solvent was removed under reduced pressure, the residue was purified by chromatography on silica gel eluted with hexanes/ethyl acetate to afford 0.2309 g (81%) of 1-cyclopropyl-4-iodopyridin-2(1H)-one.
References:
US2010/331320,2010,A1 Location in patent:Page/Page column 96

858839-90-4
89 suppliers
$52.00/250mg
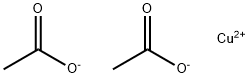
142-71-2
426 suppliers
$5.00/10g

411235-57-9
426 suppliers
$6.00/1g

1193335-00-0
2 suppliers
inquiry