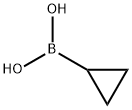
Cyclopropylboronic acid synthesis
- Product Name:Cyclopropylboronic acid
- CAS Number:411235-57-9
- Molecular formula:C3H7BO2
- Molecular Weight:85.9
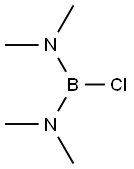
6562-41-0
10 suppliers
inquiry
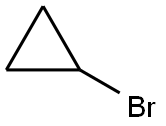
4333-56-6
377 suppliers
$6.00/5g

7732-18-5
460 suppliers
$13.50/100ML

411235-57-9
423 suppliers
$6.00/1g
Yield:411235-57-9 90%
Reaction Conditions:
Stage #1: cyclopropyl bromidewith magnesium in tetrahydrofuran at 30 - 35;Large scale;
Stage #2: chloro-bis(dimethylamino)borane in tetrahydrofuran at -10;Large scale;
Stage #3: waterwith hydrogenchloride in tetrahydrofuran;Large scale;Temperature;Solvent;
Steps:
1; 3; 5-8 Example 1
Step 1: add 200L of 2mol/L bromocyclopropane/tetrahydrofuran solution in storage tank A, add 200L of 2mol/L magnesium powder/tetrahydrofuran solution in storage tank B, add 2.5mol/L two (two (two) in storage tank C) methylamino) boron chloride/tetrahydrofuran homogeneous solution 100L, add 300kg of 1mol/L hydrochloric acid to the hydrochloric acid storage tank.Step 2: Turn on the heating device of the microreactor 1, raise the temperature to 30°C to 35°C, and send the bromocyclopropane/tetrahydrofuran solution in the storage tank A into the first block of the microreactor 1 at a flow rate of 30mL/min through the liquid delivery pump A At the same time, the magnesium powder solution in the storage tank B was stirred evenly, and sent to the first plate of the microreactor 1 at a flow rate of 30mL/min through the slurry delivery pump B; after the reaction of the 6 plates, the magnesium powder disappeared and the solution was clarified. Cool the storage tank C to -10°C, turn on the cooling device of the microreactor 2, cool down to -10°C, and pass the Grignard reagent reaction solution in the microreactor 1 into the first plate of the microreactor 2 through the channel E. The bis(dimethylamino)boron chloride tetrahydrofuran homogeneous solution in storage tank C enters the second plate of microreaction 2 through liquid delivery pump C at a flow rate of 30mL/min, and after boronization reaction of 4 plates, the hydrochloric acid is stored in the tetrahydrofuran. The hydrochloric acid in the tank is sent into the 5th plate of the microreactor 2 through the liquid delivery pump C with a flow rate of 90mL/min, and the acidification quenches the reaction;Step 3: Receive materials from the outlet of the sixth plate of microreactor 2 for sample analysis, use pinacol derivative reaction solution, organic layer GC analysis, peak area normalization results: cyclopropyl boronate pinacol ester: ring Propane >95:1, the reaction is complete. After-treatment: the collected liquid was separated into layers, the aqueous layer was extracted twice with 150 kg of ethyl acetate, the organic layers were combined, the organic layer was washed once with 60 kg of saturated brine, the organic layer was concentrated, and 45 kg of n-heptane was added. Filtration and drying of the filter cake yielded 30.92 kg of white cyclopropylboronic acid product, H-NMR content >97%, melting point 90-95°C, yield 90%.
References:
CN113735889,2021,A Location in patent:Page/Page column 5; 6-8

4333-56-6
377 suppliers
$6.00/5g
2467-18-7
6 suppliers
$172.00/250mg

7732-18-5
460 suppliers
$13.50/100ML

411235-57-9
423 suppliers
$6.00/1g
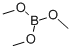
121-43-7
346 suppliers
$14.00/25mL

4333-56-6
377 suppliers
$6.00/5g

7732-18-5
460 suppliers
$13.50/100ML

411235-57-9
423 suppliers
$6.00/1g

4333-56-6
377 suppliers
$6.00/5g
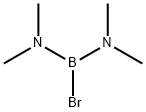
6990-27-8
7 suppliers
$281.00/10ml

7732-18-5
460 suppliers
$13.50/100ML

411235-57-9
423 suppliers
$6.00/1g

5419-55-6
370 suppliers
$12.00/5g

4333-56-6
377 suppliers
$6.00/5g

7732-18-5
460 suppliers
$13.50/100ML

411235-57-9
423 suppliers
$6.00/1g