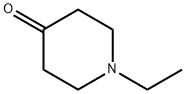
1-Ethyl-4-piperidone synthesis
- Product Name:1-Ethyl-4-piperidone
- CAS Number:3612-18-8
- Molecular formula:C7H13NO
- Molecular Weight:127.18

74-96-4
437 suppliers
$9.00/5g
41979-39-9
115 suppliers
$15.00/10mg

3612-18-8
171 suppliers
$6.00/1g
Yield:-
Reaction Conditions:
with sodium carbonate in water;acetonitrile at 85;
Steps:
17
Preparation 17: r-(Ethyl)-l,4'-bipiperidin-4-amine hydrochloride; [0128] A round bottom flask was charged with piperidin-4-one hydrochloride monohydrate (5 g, 33 mmol), acetonitrile (40 mL), bromomethane (4 g, 33 mmol), and sodium carbonate (1Og). This mixture was heated overnight at 850C, then cooled, filtered and the filtrate concentrated. The product was purified by flash chromatography (16O g column, 0-7% 2N NH3 in MeOH : DCM) to give l-(ethyl)piperidin-4-one as a yellow liquid (3.6 g, 62 % yield).To a solution of l-(ethyl)piperidin-4-one (500 mg, 3.78 mmol) and 4-(BOC amino)piperidine (757 mg, 3.78 mmol) in THF (25 mL) was added AcOH (5 drops) and sodium triacetoxyborohydride (1.2 g, 5.67 mmol). This solution was allowed to stir overnight. Water (40 mL) was then added to the reaction, the layers separated, and the organic discarded. The aqueous layer was basified with sodium carbonate (10 mL sat.) and the product extracted into EtOAc. The organic layer was dried over sodium sulfate, filtered and concentrated to give tert-butyl r-(ethyl)-l,4'-bipiperidin-4-ylcarbamate (Ig, 85 % yield). MS (ES) [M+H] found 312. tert-Butyi r-(ethyl)-l,4'-bipiperidin-4-ylcarbamate (Ig, 3.2 mmol) was dissolved in MeOH (15 mL), HCl was added (2 mL cone.) and the mixture was heated to 70 0C for 3 hours. The mixture was then cooled and to give a solid which was filtered off, washed with diethyl ether, and dried to yield the title compound (990 mg, 96% yield) as a white solid. MS (ES) [M+H] found 212.
References:
WO2010/25073,2010,A1 Location in patent:Page/Page column 29

99329-51-8
9 suppliers
$46.00/1g

3612-18-8
171 suppliers
$6.00/1g
![1,4-Dioxa-8-azaspiro[4.5]decane](/CAS/GIF/177-11-7.gif)
177-11-7
288 suppliers
$6.00/1g

3612-18-8
171 suppliers
$6.00/1g
![1,4-Dioxa-8-azaspiro[4.5]decane, 8-acetyl- (9CI)](/CAS/GIF/176910-35-3.gif)
176910-35-3
3 suppliers
inquiry

3612-18-8
171 suppliers
$6.00/1g

3619-64-5
1 suppliers
inquiry

3612-18-8
171 suppliers
$6.00/1g