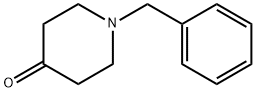
1-Benzyl-4-piperidone synthesis
- Product Name:1-Benzyl-4-piperidone
- CAS Number:3612-20-2
- Molecular formula:C12H15NO
- Molecular Weight:189.25
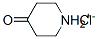
41979-39-9
116 suppliers
$15.00/10mg

100-39-0
423 suppliers
$10.00/10g

3612-20-2
485 suppliers
$6.00/10g
Yield:3612-20-2 89.28%
Reaction Conditions:
Stage #1: 4-piperidone hydrochloridewith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: benzyl bromide in N,N-dimethyl-formamide at 65; for 14 h;
Steps:
25
A mixture of 4-piperidone monohydrate hydrochloride (2.5 g, 14.56 mmol) and anhydrous potassium carbonate (7 g, 50.64 mmol) in dry DMF (25 mL) was stirred for 30 min at room temperature. Benzyl bromide (2 mL, 16.82 mmol) was added dropwise into the reaction mixture and heated at 65 °C for 14 h. The reaction mixture was cooled to room temperature, filtered and quenched with ice water (25 mL). The resulting mixture was extracted in ethyl acetate (2 x 20 mL) and the combined organic layers were washed with water (2 x 15 mL) followed by brine (20 mL). The organic phase obtained was dried over anhydrous sodium sulphate and evaporated. The crude product obtained was purified by crystallisation using 2 % methanol in chloroform to afford the title compound.Yield: 2.5 g (89.28 %); 1 HNMR (CDCI3, 300MHz): δ 7.34 (m, 4H), 7.29 (m, 1 H), 3.62 (S, 2H), 2.75 (t, 4H), 2.46 (t, 4H).
References:
WO2011/104680,2011,A1 Location in patent:Page/Page column 77-78
![3-[BENZYL-(2-METHOXYCARBONYL-ETHYL)-AMINO]-PROPIONIC ACID METHYL ESTER](/CAS/GIF/793-19-1.gif)
793-19-1
46 suppliers
$41.00/100mg

3612-20-2
485 suppliers
$6.00/10g
41661-47-6
93 suppliers
$45.00/250mg

100-39-0
423 suppliers
$10.00/10g

3612-20-2
485 suppliers
$6.00/10g
24686-78-0
140 suppliers
$6.00/1g

3612-20-2
485 suppliers
$6.00/10g

100-39-0
423 suppliers
$10.00/10g
40064-34-4
366 suppliers
$10.00/5g

3612-20-2
485 suppliers
$6.00/10g