1-fluoro-2-methylpropan-2-ol synthesis
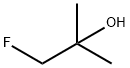
- Product Name:1-fluoro-2-methylpropan-2-ol
- CAS Number:353-80-0
- Molecular formula:C4H9FO
- Molecular Weight:92.11
Yield:353-80-0 62%
Reaction Conditions:
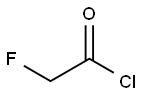
Stage #1: ethyl 2-fluoroacetate;methylmagnesium bromide in tetrahydrofuran;diethyl ether at -60 - 0; for 5 h;Inert atmosphere;
Stage #2: with hydrogenchloride;sodium chloride in tetrahydrofuran;diethyl ether;water;
Steps:
5.2.36. 1-Fluoro-2-methylpropan-2-ol (27a)
To a solution of ethyl fluoroacetate (21.2 g, 200 mmol) in tetrahydrofuran (200 mL) was added dropwise 3.0 M diethylether methyl magnesium bromide solution (128 ml, 414 mmol) at -60 °C and the reaction was stirred for 1 h under argon atmosphere. The mixture was allowed to warm to 0 °C and stirred for 4 h. To this was added water (100 mL), concd hydrochloride (36.0 mL) and sodium chloride (20.0 g), then extracted with Ether (200 mL). The organic layer was dried over sodium sulfate and distilled under normal pressure at 71-102 °C to afford a solution of 27a in tetrahydrofuran (123 mmol, 62%). 1H NMR (200 MHz, CDCl3): δ 1.25 (3H, s), 1.26 (3H, s), 4.20 (2H, d, J = 47.5 Hz).
References:
Chonan, Tomomichi;Wakasugi, Daisuke;Yamamoto, Daisuke;Yashiro, Miyoko;Oi, Takahiro;Tanaka, Hiroaki;Ohoka-Sugita, Ayumi;Io, Fusayo;Koretsune, Hiroko;Hiratate, Akira [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 5,p. 1580 - 1593] Location in patent:experimental part