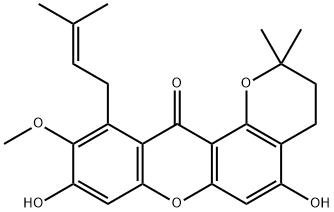
1-Isomangostin synthesis
- Product Name:1-Isomangostin
- CAS Number:19275-44-6
- Molecular formula:C24H26O6
- Molecular Weight:410.46
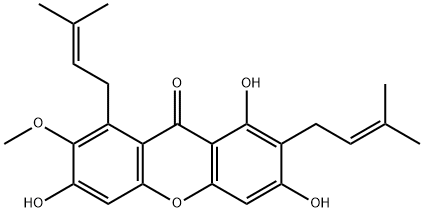
6147-11-1
274 suppliers
$39.00/10mg
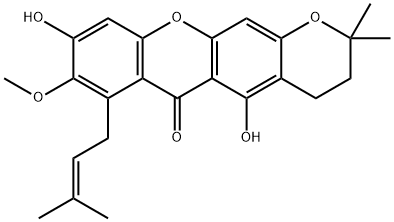
19275-46-8
120 suppliers
$198.00/10mg

19275-44-6
25 suppliers
inquiry
Yield:19275-46-8 65% ,19275-44-6 15%
Reaction Conditions:
with toluene-4-sulfonic acid in benzene; for 0.5 h;Reflux;Dean-Stark;
Steps:
3-isomangostin (4a)5C and 1-isomangostin.
A mixture of a-mangostin (150.0 mg, 0.37 mmol) and PTSA (172.0 mg) in dry benzene (11 mL) was refluxed over a water bath with a Dean-Stark apparatus for a 30 mins. Benzene was removed, and the residue was extracted with CHCl3 (3 x 50 mL). The organic layer was washed with H2O (2 x 50 mL) and dried with anhydrous over anhydrous MgSO4. Chromatography over silica gel with 15% aceteone-hexane as an eluent yielded of 4a (98.7 mg, 65% yield; Rf = 0.53 (20% acetone-hexane)) and 4a-1 (22.7 mg, 15% yield; Rf = 0.71 (20% acetone-hexane)). 3-isomangostin (4a) Yellow powder (m.p. 164-166 °C). 1H NMR 300 MHz (CDCl3) d (ppm) 13.72 (1H, s, 1-OH), 6.82 (1H, s, H-5), 6.22 (1H, s, H-4), 5.27 (1H, br t, J = 6.6 Hz, H-2¢¢), 4.09 (2H, d, J = 6.6 Hz, H-1¢¢), 1.85 (3H, s, CH3-5¢¢), 1.69 (3H, s, CH3-4¢¢), 2.71 (2H, t, J = 6.9 Hz, H-1¢), 1.85-1.81 (2H, m, H-2¢), 1.37 (6H, s, CH3-4¢ and CH3-5¢), 3.80 (3H, s, 7-OCH3). 1-isomangostin (4a-1). Yellow powder (m.p. 164-166 °C). 1H NMR 300 MHz (CDCl3) d (ppm) 6.82 (1H, s, H-5), 6.22 (1H, s, H-4), 5.27 (1H, br t, J = 6.6 Hz, H-2¢¢), 4.09 (2H, d, J = 6.6 Hz, H-1¢¢), 1.85 (3H, s, CH3-5¢¢), 1.69 (3H, s, CH3-4¢¢), 2.67 (2H, t, J = 6.9 Hz, H-1¢), 1.85-1.81 (2H, m, H-2¢), 1.42 (6H, s, CH3-4¢ and CH3-5¢), 3.80 (3H, s, 7-OCH3).
References:
Boonnak, Nawong;Chantrapromma, Suchada;Kaewpiboon, Chutima;Sathirakul, Korbtham [Bioorganic and Medicinal Chemistry Letters,2020,vol. 30,# 20,art. no. 127494] Location in patent:supporting information

6147-11-1
274 suppliers
$39.00/10mg

26063-96-7
46 suppliers
inquiry

26063-95-6
42 suppliers
inquiry

19275-46-8
120 suppliers
$198.00/10mg

19275-44-6
25 suppliers
inquiry

6147-11-1
274 suppliers
$39.00/10mg

26063-96-7
46 suppliers
inquiry

26063-95-6
42 suppliers
inquiry

19275-44-6
25 suppliers
inquiry

6147-11-1
274 suppliers
$39.00/10mg

19275-46-8
120 suppliers
$198.00/10mg

19275-44-6
25 suppliers
inquiry
![5H,10H-Dipyrano[3,2-b:4',3',2'-kl]xanthen-5-one, 2,3,11,12-tetrahydro-13-hydroxy-4-methoxy-10,10-dimethyl-2-(1-methylethyl)-](/CAS/20210305/GIF/207984-92-7.gif)
207984-92-7
0 suppliers
inquiry