
1-isopentyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione synthesis
- Product Name:1-isopentyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione
- CAS Number:565448-79-5
- Molecular formula:C12H14N2O3
- Molecular Weight:234.25
![1H-pyrido[2,3-d][1,3]oxazine-2,4-dione](/CAS2/GIF/21038-63-1.gif)
21038-63-1
30 suppliers
$62.00/100mg
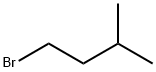
107-82-4
271 suppliers
$13.00/25g
![1-isopentyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione](/CAS2/GIF/565448-79-5.gif)
565448-79-5
8 suppliers
inquiry
Yield:565448-79-5 95%
Reaction Conditions:
Stage #1: 3-azaisatoic anhydridewith sodium hydride in ISOPROPYLAMIDE at 20; for 0.5 h;
Stage #2: i-pentyl bromide in ISOPROPYLAMIDE; for 16 h;
Steps:
12A EXAMPLE 12 3-(1,1-dioxido-4H-1,2,4-benzothiadiazin-3-yl)-4-hydroxy-1-(3-methylbutyl)-1,8-naphthyridin-2(1H)-one EXAMPLE 12A 1-(3-methylbutyl)-2H-pyrido[2,3-d][1,3]oxazine-2,4(1H)-dione
1-(3-methylbutyl)-2H-pyrido[2,3-d][1,3]oxazine-2,4(1H)-dione A suspension of sodium hydride (95%, 0.048 g, 2.0 mmol) in dimethylacetamide (2 ML) at 20° C. under nitrogen was reacted with the product of Example 1A (0.3 g, 1.83 mmol).The reaction mixture was stirred for 1/2 hour then treated with 1-bromo-3-methylbutane (0.3 g, 2.0 mmol) and stirred for an additional 16 hours.The reaction was partitioned between ethyl acetate and water.The organic layer was washed with water and brine, dried over sodium sulfate, filtered and concentrated under vacuum.The crude product was purified by flash column chromatography on silica gel eluding with hexanes and ethyl acetate (3:1) to give the title compound as a white solid (0.218 g, 51%). MS (ESI-) m/z 233 (M-H)-. 1H NMR (300 MHz, DMSO-d6) δ 0.93 (s, 3H), 0.96 (s, 3H), 1.55 (m, 2H), 1.66 (m, 1H), 4.14 (t, J=7.72 Hz, 2H), 7.37 (dd, J=7.91, 4.96 Hz, 1H), 8.38 (dd, J=7.72, 1.84 Hz, 1H), 8.78 (dd, J=4.78, 1.84 Hz, 1H).
References:
US2004/87577,2004,A1 Location in patent:Page 40