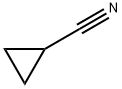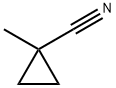
1-methylcyclopropane-1-carbonitrile synthesis
- Product Name:1-methylcyclopropane-1-carbonitrile
- CAS Number:78104-88-8
- Molecular formula:C5H7N
- Molecular Weight:81.12
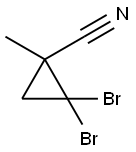
63262-97-5
0 suppliers
inquiry

78104-88-8
12 suppliers
inquiry
Yield:78104-88-8 95.7%
Reaction Conditions:
with sodium in ethanol;water;toluene at 20; for 1 h;Inert atmosphere;
Steps:
1.2
General procedure: To a 500 ml three-neck round bottom flask equipped with a mechanical stirrer,put into a6.9 g sodium and120 ml of anhydrous toluene,The oil bath is heated to the metal sodium to melt,Stir quickly to form sodium sand.Under the conditions of nitrogen extraction of toluene,Then addedToluene was washed off with 100 ml of anhydrous tetrahydrofuran,Add to the reaction flask15.0g2,2-dichloro-1-methylcyclopropanecarbonitrile (0.1 mol)Thirty milliliters of ethanol / water (19/2) mixture was added dropwise.After completion of the dropwise addition,The mixture was stirred at room temperature for 1 hour,Extracted with methylene chloride,dry,The solvent was removed by evaporation,7.5 g of 1-methylcyclopropanecarbonitrile was obtained,The yield was 92.6%Purity & gt; 95%. Using 2,2-dibromo-1-methylcyclopropanecarbonitrile in place of 2,2-dichloro-1-methylcyclopropanecarbonitrile, the same procedure gave 1-methylcyclopropanecarbonitrile in a yield of 95.7% and a purity of & gt; 95%.
References:
CN104447293,2016,B Location in patent:Paragraph 0039; 0040
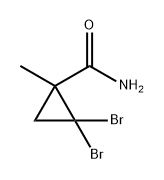
122665-05-8
0 suppliers
inquiry

78104-88-8
12 suppliers
inquiry
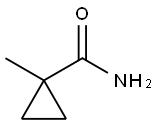
15910-91-5
51 suppliers
$45.00/1mg

78104-88-8
12 suppliers
inquiry
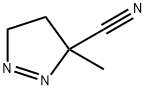
6116-90-1
0 suppliers
inquiry

78104-88-8
12 suppliers
inquiry