
1-N-BOC-4-(2-CHLORO-4-PYRIDINYL) PIPERAZINE synthesis
- Product Name:1-N-BOC-4-(2-CHLORO-4-PYRIDINYL) PIPERAZINE
- CAS Number:633283-63-3
- Molecular formula:C14H20ClN3O2
- Molecular Weight:297.78
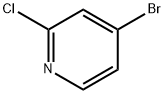
73583-37-6
318 suppliers
$9.00/1g

57260-71-6
732 suppliers
$5.00/5g

633283-63-3
26 suppliers
inquiry
Yield:633283-63-3 100%
Reaction Conditions:
with sodium t-butanolate;tris-(dibenzylideneacetone)dipalladium(0);4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in toluene at 85; for 20 h;Inert atmosphere;
Steps:
16
xample 16: N-CyclopentyW-piperazin-i-ylpyridin^-amine. 4-(2-Chloro-pyridin-4-yl)-piperazine-1 -carboxylic acid tert-butyl ester. To a stirring solution of 2-chloro-4-bromopyridine (4.3 g, 22.5 mmol) in toluene (100 ml_) was added piperazine-1-carboxylic acid tert-butyl ester (3.2 g, 17.2 mmol) and sodium tert-butoxide (2.5 g, 26.0 mmol). The flask was evacuated and flushed with N2(g) twice. A mixture of Xantphos (600 mg, 1.0 mmol) and Pd2(dba)3 (318 mg, 0.35 mmol) was added in one portion and the mixture was heated at 85 0C for 20 h. The mixture was cooled to rt, diluted with H2O (75 -->ml_), and extracted with EtOAc (3x). The combined organic layers were dried and concentrated to give a clear golden oil. The oil was purified on FCC (O to 5% 2 M NH3 in MeOH/CH2CI2) to give the title product as a beige solid (5.1 g, 100%). MS (ESI): mass calcd. for Ci4H20CIN3O2, 297.1 ; m/z found, 298.2 [M+H]+. 1H NMR (DMSO-d6): 7.95 (d, J = 5.9, 1 H), 6.88-6.78 (m, 2H), 3.46- 3.32 (m, 8H), 1.42 (s, 9H). terf-Butyl 4-[2-(cyclopentylamino)pyridin-4-yl]piperazine-1 -carboxylate. To a stirring solution of 4-(2-chloro-pyridin-4-yl)-piperazine-1-carboxylic acid tert- butyl ester (163 mg, 0.55 mmol) in toluene (2 ml_) in a vial was added cyclopentylamine (136 μl_, 1.38 mmol) and sodium tert-butoxide (161 mg, 1 .68 mmol). A mixture of racemic BINAP (20 mg, 0.032 mmol) and Pd(OAc)2 (18 mg, 0.027 mmol) was added in one portion and the mixture was heated at 85 0C for 20 h. The mixture was cooled to rt and purified directly by FCC (0 to 5% 2 M NH3 in MeOH/CH2CI2) to provide the title compound as a white solid (34 mg, 17%). MS (ESI): mass calcd. for Ci9H30N4O2, 346.2; m/z found, 347.3 [M+H]+. 1H NMR (DMSO-d6): 7.65 (d, J = 6.0, 1 H), 6.10 (d, J = 6.0, 1 H), 5.92 (d, J = 7.1 , 1 H), 5.78 (s, 1 H) 4.08-4.02 (m, 1 H), 3.41 (t, J = 5.4, 4H), 3.16 (t, J = 5.4, 4H), 1.89-1.82 (m, 2H), 1.67-1 .61 (m, 2H), 1 .59-1.48 (m, 2H), 1.42 (s, 9H), 1.43-1.35 (m, 2H). N-Cyclopentyl-4-piperazin-1-ylpyridin-2-amine dihydrochloride. To a stirring solution of tert-butyl 4-[2-(cyclopentylamino)pyhdin-4-yl]piperazine-1 - carboxylate (34 mg, 0.1 mmol) in 96% formic acid (4 ml_) was added 6 N aq HCI (2 drops). The mixture was stirred for 2 h and concentrated to give the desired product as a white solid (26 mg, 93%). MS (ESI): mass calcd. for Ci4H22N4, 246.2; m/z found, 247.2 [M+H]+. 1H NMR (DMSO-d6): 9.32 (s, 2H), 7.89 (d, J = 7.1 , 1 H), 7.69 (d, J = 7.5, 1 H), 6.58 (d, J = 7.6, 1 H), 6.04 (s, 1 H), 4.03-3.96 (m, 1 H), 3.73 (t, J = 4.9, 4H), 3.20 (t, J = 5.0, 4H), 2.03-1.95 (m, 2H), 1.73-1.68 (m, 2H), 1.64-1 .52 (m, 2H), 1.50-1.42 (M, 2H).
References:
WO2009/152325,2009,A1 Location in patent:Page/Page column 47-48
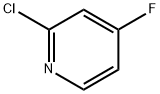
34941-91-8
251 suppliers
$5.00/1g

57260-71-6
732 suppliers
$5.00/5g

633283-63-3
26 suppliers
inquiry
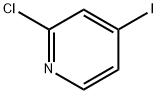
153034-86-7
364 suppliers
$10.00/1g

57260-71-6
732 suppliers
$5.00/5g

633283-63-3
26 suppliers
inquiry