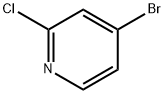
2-Chloro-4-bromopyridine synthesis
- Product Name:2-Chloro-4-bromopyridine
- CAS Number:73583-37-6
- Molecular formula:C5H3BrClN
- Molecular Weight:192.44
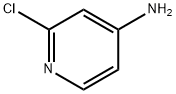
14432-12-3
530 suppliers
$5.00/5g

73583-37-6
322 suppliers
$9.00/1g
Yield:73583-37-6 96%
Reaction Conditions:
with tert.-butylnitrite;copper(ll) bromide in acetonitrile at 0 - 20; for 16 h;regioselective reaction;Sandmeyer Reaction;
Steps:
General procedure for the synthesis of substrates
General procedure: To a mixture of copper source (1.2 eq., CuBr2 for 4b, CuCl2 for 4c, CuI for 4d)in acetonitrile (0.4M) was slowly added tBuONO (1.5 eq.). The mixture was stirred for15 min and then cooled to 0°C. A solution of 2-chloro-4-aminopyridine 3 (1.0 eq.) inacetonitrile (0.5M) was slowly added. The mixture was stirred for 1h at 0°C and thenallowed to warm to r.t. for 16h. After concentration in vacuo, aq. 15% ammonia solution (1.3 mL/mmol) was added, and the aqueous layer was extracted withdichloromethane (10 mL/mmol; 3x). The combined organic layers were washed withbrine, dried (Na2SO4), filtered and carefully concentrated under reduced pressure(CAUTION: volatile products) to give the crude 4b-d, which was used in the next stepwithout purification.
References:
Honraedt, Aurélien;Gallagher, Timothy [Synlett,2016,vol. 27,# 1,art. no. ST-2015-D0455-L,p. 67 - 69] Location in patent:supporting information
30766-03-1
265 suppliers
$7.00/1g

73583-37-6
322 suppliers
$9.00/1g
52200-48-3
360 suppliers
$11.00/1g

73583-37-6
322 suppliers
$9.00/1g
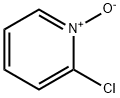
2402-95-1
178 suppliers
$55.00/1g

73583-37-6
322 suppliers
$9.00/1g
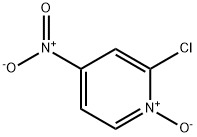
14432-16-7
213 suppliers
$10.00/1g

73583-37-6
322 suppliers
$9.00/1g