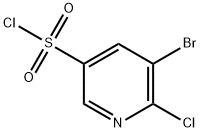
5-Bromo-6-chloropyridine-3-sulfonyl chloride synthesis
- Product Name:5-Bromo-6-chloropyridine-3-sulfonyl chloride
- CAS Number:216394-05-7
- Molecular formula:C5H2BrCl2NO2S
- Molecular Weight:290.95
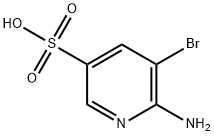
247582-62-3
75 suppliers
$48.00/250 mg

216394-05-7
101 suppliers
$36.25/1g
Yield:216394-05-7 100%
Reaction Conditions:
Stage #1:2-Amino-3-bromopyridine-5-sulphonic Acid with hydrogenchloride;sodium nitrite in water at 0; for 1 h;
Stage #2: with phosphorus pentachloride;trichlorophosphate at 75 - 125;
Steps:
199.1
A cooled (0 0C) solution of θ-amino-S-bromopyridine-S-sulfonic acid (12.65 g; 50.00 mmol) in HCI (60 ml; 5 N solution in water) was treated carefully with a solution of sodium nitrite (3.80 g; 55.0 mmol) in water (15 ml) and stirred at 0 0C for 1 hour. The solvents were evaporated and the residue was dried under high vacuum for 2 days, then treated with phosphorus pentachloride (15.00 g; 72 mmol) and phosphorus oxide chloride (0.50 ml; 5.5 mmol). The solid mixture was heated at 125 0C to give a refluxing solution. After heating at 75 0C for 3 hours, the solution was cooled and carefully poured on crushed ice. EtOAc was added and the phases separated. The organic phase was washed with brine, dried on MgSO4, filtered and concentrated under reduced pressure to give the title compound as a brown oil (14.91 g, quantitative yield), which was used without purification.
References:
MERCK SERONO S.A.;CROSIGNANI, Stefano;JORAND-LEBRUN, Catherine;CLEVA, Christophe;PRETRE, Adeline WO2010/92043, 2010, A1 Location in patent:Page/Page column 145-146
504-29-0
539 suppliers
$5.00/5 g

216394-05-7
101 suppliers
$36.25/1g
16250-08-1
73 suppliers
$14.00/250mg

216394-05-7
101 suppliers
$36.25/1g