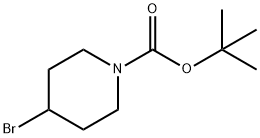
1-N-BOC-4-BROMOPIPERIDINE synthesis
- Product Name:1-N-BOC-4-BROMOPIPERIDINE
- CAS Number:180695-79-8
- Molecular formula:C10H18BrNO2
- Molecular Weight:264.16

24424-99-5
833 suppliers
$13.50/25G
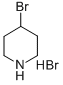
54288-70-9
157 suppliers
$7.00/1g

180695-79-8
192 suppliers
$14.00/1g
Yield:180695-79-8 100%
Reaction Conditions:
Stage #1: 4-bromopiperidine hydrobromidewith N-ethyl-N,N-diisopropylamine in dichloromethane at 0;
Stage #2: di-tert-butyl dicarbonate in dichloromethane at 20;
Steps:
G.1
Step 1: Synthesis of 4-Bromo-piperidine-1-carboxylic acid tert-butyl ester To a suspension of 5 g (0.02 mol) of 4-bromopiperidine hydrobromide salt in DCM (35 mL) are added 7.09 mL (0.04 mol) of N,N-diisopropylethyl amine dropwise at 0° C. The reaction mixture is stirred for 30 min, then a solution of 6.67 g (0.31 mol) of di-tert-butyl dicarbonate in DCM (35 mL) is added dropwise to the reaction mixture. The reaction mixture is stirred for 18 h at room temperature, then washed with 1M aqueous HCl solution (2*30 mL) and brine (30 mL). The organic layer is dried over Na2SO4, filtered and the filtrate is concentrated under reduced pressure to afford 6.9 g of 4-bromo-piperidine-l-carboxylic acid tert-butyl ester as a yellow oil. Yield quantitative; 1H NMR (250 MHz, CHLOROFORM-d) δ ppm 1.46 (9H, s), 1.79-2.00 (2H, m), 2.00-2.16 (2H, m), 3.31 (2H, ddd, J=13.67, 7.73, 3.73 Hz), 3.68 (2H, ddd, J=13.55, 6.85, 3.65 Hz), 4.34 (1H, tt, J=7.69, 3.81 Hz)
References:
US2010/76029,2010,A1 Location in patent:Page/Page column 42

24424-99-5
833 suppliers
$13.50/25G

180695-79-8
192 suppliers
$14.00/1g

109384-19-2
470 suppliers
$5.00/1g

180695-79-8
192 suppliers
$14.00/1g
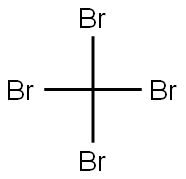
558-13-4
254 suppliers
$10.00/1g

109384-19-2
470 suppliers
$5.00/1g

180695-79-8
192 suppliers
$14.00/1g
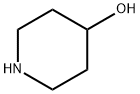
5382-16-1
13 suppliers
$9.00/5G

180695-79-8
192 suppliers
$14.00/1g