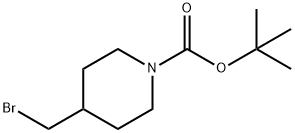
4-Bromomethypiperidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:4-Bromomethypiperidine-1-carboxylic acid tert-butyl ester
- CAS Number:158407-04-6
- Molecular formula:C11H20BrNO2
- Molecular Weight:278.19
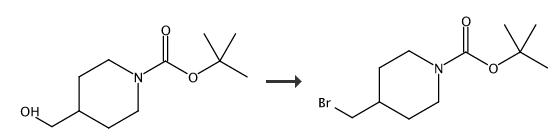
4-Bromomethypiperidine-1-carboxylic acid tert-butyl ester. 4-N-Boc-piperidine-methanol (200 mg, 0.93 mmol) was dissolved in diethyl ether (9 mL) and carbon tetrabromide (370 mg, 1.1 mmol) and PPh3 (292 mg, 1.1 mmol) were added at rt. The reaction was allowed to stir for 18 h at rt and filtered over a pad of celite. The filtrate was concentrated and purified by flash chromatography (hexane/EtOAc, 1:0 → 4:1) to give the title compound. Yield 55 mg. 1 (400 MHz, DMSO-d6) δ ppm 4.02-3.98 (m, 2 H), 3.47 (d, 2 H), 2.78-2.65 (m, 2 H), 1.89-1.74 (m, 3 H), 1.45 (s, 9 H), 1.12-0.98 (m, 2 H).
123855-51-6
418 suppliers
$6.00/5g

158407-04-6
179 suppliers
$5.00/100mg
Yield:158407-04-6 94%
Reaction Conditions:
with carbon tetrabromide;triphenylphosphine in dichloromethane at 0 - 20; for 22 h;
Steps:
14
Compound 14; EPO
References:
CURIS, INC. WO2006/50506, 2006, A1 Location in patent:Page/Page column 120-121

161975-39-9
117 suppliers
$12.00/250mg

158407-04-6
179 suppliers
$5.00/100mg
84358-13-4
390 suppliers
$5.00/5g

158407-04-6
179 suppliers
$5.00/100mg
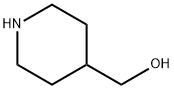
6457-49-4
387 suppliers
$18.00/25g

158407-04-6
179 suppliers
$5.00/100mg

24424-99-5
821 suppliers
$13.50/25G

158407-04-6
179 suppliers
$5.00/100mg