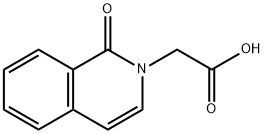
(1-Oxo-1H-isoquinolin-2-yl)-acetic acid synthesis
- Product Name:(1-Oxo-1H-isoquinolin-2-yl)-acetic acid
- CAS Number:59139-93-4
- Molecular formula:C11H9NO3
- Molecular Weight:203.19
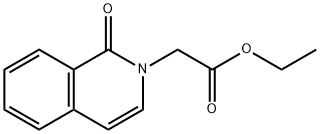
59139-95-6
0 suppliers
inquiry

59139-93-4
35 suppliers
$68.00/100mg
Yield:59139-93-4 98%
Reaction Conditions:
with sodium hydroxide in methanol;water at 20;
Steps:
A suspension of Intermediate k (1 g, 4.3 mmol) in methanol(30 mL) was treated with 2 M NaOH (15 mL) and the reactionmixture was stirred overnight at rt. Reaction mixture wasconcentrated at reduced pressure. The completion of reaction wasmonitored by TLC. Residue was diluted with water followed byadding aqueous 10% HCl to adjust pH to 5e6. Aqueous layer wasextracted with ethyl acetate. Combined organic layer was driedover anhydrous sodium sulfate. The solvent was evaporated invacuo and purified by chromatographic purification with a petroleumether/ethyl acetate gradient to obtain 2-(1-oxoisoquinolin-2(1H)-yl)acetic acid (m) as a white solid (0.855 g, 98% yield). 1HNMR (400 MHz, DMSO-d6) d 8.21 (d, J 8.0 Hz, 1H), 7.75e7.69 (m,1H), 7.66 (d, J 7.8 Hz, 1H), 7.55e7.48 (m, 1H), 7.43 (d, J 7.4 Hz,1H), 6.62 (d, J 7.4 Hz, 1H), 4.66 (s, 2H).
References:
Jiao, Yan;Nan, Jinshan;Mu, Bo;Zhang, Yun;Zhou, Nenghua;Yang, Shunhua;Zhang, Shanshan;Lin, Wanting;Wang, Falu;Xia, Anjie;Cao, Zhixing;Chen, Pei;Pan, Zhiling;Lin, Guifeng;Pan, Shulei;Bin, Huachao;Li, Linli;Yang, Shengyong [European Journal of Medicinal Chemistry,2022,vol. 232,art. no. 114194]

1444010-78-9
0 suppliers
inquiry

59139-93-4
35 suppliers
$68.00/100mg
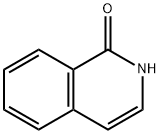
491-30-5
261 suppliers
$7.00/250mg

59139-93-4
35 suppliers
$68.00/100mg
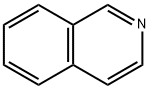
119-65-3
404 suppliers
$5.00/25g

59139-93-4
35 suppliers
$68.00/100mg
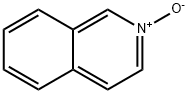
1532-72-5
162 suppliers
$15.00/1g

59139-93-4
35 suppliers
$68.00/100mg