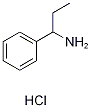
1-phenyl-1-propanamine hydrochloride synthesis
- Product Name:1-phenyl-1-propanamine hydrochloride
- CAS Number:24301-86-8
- Molecular formula:C9H14ClN
- Molecular Weight:171.6672
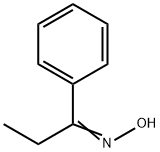
2157-50-8
8 suppliers
inquiry

24301-86-8
11 suppliers
$110.00/50mg
Yield:-
Reaction Conditions:
with hydrogenchloride;hydrogen;palladium 10% on activated carbon in ethanol;water; under 760.051 Torr;
Steps:
72
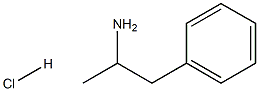
First step, hydroxyamine hydrochloride (5.15 g) and NaOAc (12.23 g) were added to a solution of 1-propiophenone (5 g) in EtOH (80 ml). The reaction mixture was stirred at 60°C for 6 h. EtOH was removed under reduced pressure. H2O (40 ml) was added to the residue, and the resulting solution was extracted with EtOAc (3 × 40 ml). The EtOAc of the combined organic layer was removed by rotary evaporation under reduced pressure to yield 1-propiophenone oxime (4.75 g) as a pale yellowish solid. Second step, a solution of 1-propiophenone oxime (4.75 g) and concentrated HCl (16.8 ml) in EtOH (50 ml) was subjected to hydrogenation at atmospheric pressure in the presence of 10% Pd/C (674 mg). The reaction solution was filtered and the filtrate was concentrated to yield 1-(phenyl)-propylamine hydrochloride (5.40 g) as a white solid. Third step, a mixture of 1-(phenyl)-propylamine (359 mg, the hydrochloride), 6-chloropurine riboside (200 mg) and triethylamine (3 ml) in PrOH (60 ml) was heated to 70°C and reacted for 8 h. After evaporation of the reaction mixture, the residue was separated by column chromatography over silica gel and eluted with CHCl3-CH3OH (20: 1) to yield N6-[(+/-)-1-(phenyl)-propyl]-adenosine(215 mg) as a white solid: positive ESIMS mlz 386 [M + H]+: negative ESIMS mlz 420 [M + Cl]-; 1H NMR (300 MHz, DMSO-d6): the adenosine moiety δ 8.36 (1H, s, H-2), 8.31 (1H, brs, -NH), 8.14 (1H, s, H-8), 5.86 (1H, d, J= 6.0 Hz, H-1'), 5.40 (1H, m, -OH), 5.36 (1H, m, -OH), 5.15 (1H, d, J = 4.5 Hz, -OH), 4.58 (1H, m, H-2'), 4.12 (1H, m, H-3'), 3.94 (1H, m, H-4'), 3.66 (1H, m, H-5'a), 3.56 (1H, m, H-5'b); the (+/-)-1-(phenyl)-propyl moiety δ 7.44 (2H, d, J = 7.2 Hz, H-2", H-6"), 7.27 (2H, t, J = 7.4 Hz, H-3", H-5"), 7.18 (1H, t, J = 7.4 Hz, H-4"), 5.25 (1H, m, H-7"), 1.95 (1H, m, H-8"a), 1.83 (1H, m, H-8"b), 0.89 (3H, t, J = 6.6 Hz, H-9"); 13C NMR (75 MHz, DMSO-d6): the adenosine moiety δ 154.3 (s, C-6), 152.2 (d, C-2), 148.4 (s, C-4), 139.7 (d, C-8), 119.7 (s, C-5), 87.9 (d, C-1'), 85.9 (d, C-4'), 73.4 (d, C-2'), 70.6 (d, C-3'), 61.6 (t, C-5'); the (+/-)-1-(phenyl)-propyl moiety δ 144.2 (s, C-1"), 128.1 (d, C-2", C-6"), 126.7 (d, C-3", C-5"), 126.6 (d, C-4"), 55.2 (d, C-7"), 29.0 (t, C-8"), 11.5 (q, C-9").
References:
EP2511283,2012,A1 Location in patent:Page/Page column 77

103-65-1
173 suppliers
$18.00/25mL
2706-50-5
0 suppliers
$18.00/1mg

24301-86-8
11 suppliers
$110.00/50mg
189387-89-1
0 suppliers
inquiry

24301-86-8
11 suppliers
$110.00/50mg
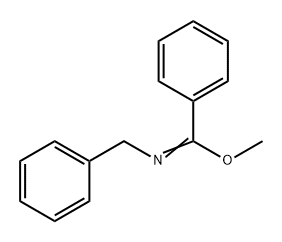
79893-76-8
0 suppliers
inquiry

24301-86-8
11 suppliers
$110.00/50mg
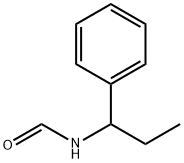
83834-93-9
0 suppliers
inquiry

24301-86-8
11 suppliers
$110.00/50mg