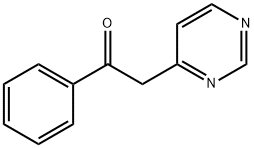
1-PHENYL-2-(4-PYRIMIDINYL)-ETHANONE synthesis
- Product Name:1-PHENYL-2-(4-PYRIMIDINYL)-ETHANONE
- CAS Number:36912-83-1
- Molecular formula:C12H10N2O
- Molecular Weight:198.22
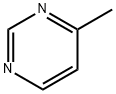
3438-46-8
224 suppliers
$9.00/5g
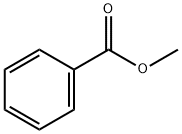
93-58-3
468 suppliers
$14.00/25mL

36912-83-1
31 suppliers
$57.00/1g
Yield:36912-83-1 88%
Reaction Conditions:
with potassium tert-butylate in N,N-dimethyl-formamide at 50; for 1 h;
Steps:
1-Phenyl-2-(pyrimidin-4-yl)ethan-1-one (10a)
To a solution of 4-methylpyrimidine (4) (128.0 g, 1.360 mol) and methyl benzoate (222.2 g, 1.632 mol)in DMF (640 mL) was added potassium t-butoxide (229.1 g, 2.042 mol), and the mixture was stirred at50 °C for 1 h under dry conditions. After cooling to 10 °C or less, 2 mol/L HCl aqueous solution (1000mL) was added until pH 5~6. The mixture was poured into ice-cold water (2550 mL), and the resultingprecipitate was collected by filtration. The precipitate was washed with 0.8 mol/L NaHCO3 aqueoussolution (1280 mL) and water (1280 mL), and forced-air dried at 60 °C to provide the title compound 10a(237.4 g, 88%) as a yellow solid
References:
Ohta, Shuji;Saito, Takahisa;Kato, Jun-Ya;Sato, Shuichiro;Hayashi, Hiroyuki;Hasumi, Koichi [Heterocycles,2017,vol. 94,# 5,p. 938 - 948]

