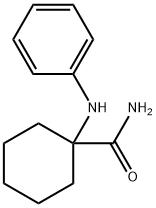
1-(phenylamino)cyclohexanecarboxamide synthesis
- Product Name:1-(phenylamino)cyclohexanecarboxamide
- CAS Number:64269-12-1
- Molecular formula:C13H18N2O
- Molecular Weight:218.29
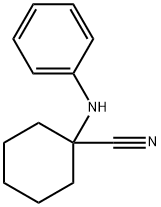
64269-06-3
19 suppliers
inquiry

64269-12-1
14 suppliers
$35.35/250mgs:
Yield:64269-12-1 95%
Reaction Conditions:
Stage #1: 1-(phenylamino)cyclohexanecarbonitrilewith sulfuric acid at 20; for 48 h;
Stage #2: with sodium hydroxide;water at 0; pH=7 - 8;
Steps:
2.a
Example 2N-(2,6-Diisopropylphenyl)-2-(1 -phenyl-1 ,3-diazaspiro[4.5]dec-3-yl)- acetamide, compound (1.2); With R = H; R1 = R2 = iPr; -R3-R6- = -CH2-; R4 and R4 are bonded to one another to form a cyclohexyl; R5 = Pha/ 1 -Phenylaminocyclohexanecarboxylamide.5 g (25 mmol) of 1 -phenylaminocyclohexanecarbonitrile (obtained according to Example 1 a) are dissolved in 30 ml of concentrated sulphuric acid. The reaction medium is stirred at room temperature for 48 h. It is then gently poured into ice and the pH is set at 7-8 with sodium hydroxide and the mixture is extracted with ethyl acetate. The organic phases are collected and washed with water. They are dried over sodium sulphate. The solvents are evaporated and the residue is precipitated in dichloromethane and heptane and then filtered and dried.5.2 g of 1 -phenylaminocyclohexanecarboxylamide are obtained in the form of a white solid. M. p. 146-8°C, yield = 95%.
References:
WO2009/30750,2009,A1 Location in patent:Page/Page column 30

108-94-1
528 suppliers
$12.00/50g

64269-12-1
14 suppliers
$35.35/250mgs:

62-53-3
642 suppliers
$10.00/1g

64269-12-1
14 suppliers
$35.35/250mgs:
142-04-1
185 suppliers
$10.00/10g

64269-12-1
14 suppliers
$35.35/250mgs: