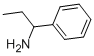
1-Phenylpropan-1-amine synthesis
- Product Name:1-Phenylpropan-1-amine
- CAS Number:2941-20-0
- Molecular formula:C9H13N
- Molecular Weight:135.21
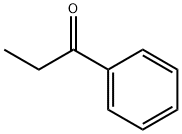
93-55-0
0 suppliers
$15.00/25g

2941-20-0
69 suppliers
$16.00/500mg
Yield: 94%
Reaction Conditions:
with {RuCl(p-cymene)((S)-2,2'-bis(di-p-tolylphosphino)-1,1'-binaphthyl)}Cl;ammonia;hydrogen;ammonium chloride in methanol;water at 90; under 30003 Torr; for 48 h;Autoclave;Inert atmosphere;enantioselective reaction;Reagent/catalyst;
Steps:
10
General procedure: General procedure for the asymmetric reductive amination using ammonium chloride as the ammonium salt.All manipulations were done under an atmosphere of nitrogen. The methanol and water were deoxygenated by 5 vacuum/nitrogen cycles prior to use.The reactions were carried out in small autoclaves that can be pressurized to 50 bar. To a 2OmL glass vial is added immol of the oxime, 0,O2Smmol of preformed catalyst, lOmmol NH4CI and 2mL degassed methanol, immol NH3 (added as a 7M solution in methanol) and 20iiL water. The glass vial is put into the parallel autoclave under an atmosphere of nitrogen gas and 40 bar of hydrogen is applied and the autoclave is heated to 90°C and agitated. After 48 hours the autoclave is cooled to 20°C and then the hydrogen pressure is released. The methanol is evaporated off and NaOH(aq) is added to the residue to pH>11. The mixture is extracted with diethylether. The combined ether fractions are dried over MgSO4, filtered and concentrated under vacuum to afford the amine product. The products were analyzed by chiral HPLC and 1H and 13C NMR spectroscopy and were in accordance with authentic samples.
References:
SP PROCESS DEVELOPMENT AB;RYBERG, Per;BERG, Robert WO2015/178846, 2015, A1 Location in patent:Page/Page column 5; 6
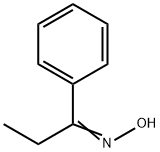
2157-50-8
7 suppliers
inquiry

2941-20-0
69 suppliers
$16.00/500mg
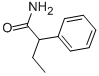
90-26-6
93 suppliers
$34.39/100g

2941-20-0
69 suppliers
$16.00/500mg
93-54-9
247 suppliers
$6.00/5g

2941-20-0
69 suppliers
$16.00/500mg

934-11-2
19 suppliers
$45.00/10mg

2941-20-0
69 suppliers
$16.00/500mg