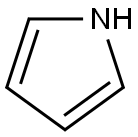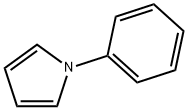
1-PHENYLPYRROLE synthesis
- Product Name:1-PHENYLPYRROLE
- CAS Number:635-90-5
- Molecular formula:C10H9N
- Molecular Weight:143.19
Yield:635-90-5 78%
Reaction Conditions:
with sodium t-butanolate in ethylene glycol at 100; for 6 h;Inert atmosphere;Green chemistry;
Steps:
2.2.8 General procedure for the N-arylation of nitrogen-containing compounds with C-O activation of aryl carbamate and/or aryl sulfamate
General procedure: Aryl carbamate and/or aryl sulfamate (1.0mmol), nitrogen-containing compounds (1.0mmol), sodium tert-butoxide (2.0mmol), Fe3O4SiO2-EDTA-Ni(II) NPs (0.018g, 1mol %) and ethylene glycol (3.0mL) were added into a round-bottomed flask and stirred at 100°C for 12h under the inert nitrogen atmosphere. The reaction progress was monitored by TLC using petroleum ether/ethyl acetate and/or GC (argon in 5.0grades or 99.999% purity as carrier gas and PEG as stationary phase). After the completion of the reaction, the reaction mixture was cooled to room temperature and the nanocatalysts separated from the mixture with an external magnetic field. Then, water (10mL) was added and the crude mixture was subsequently extracted with ethyl acetate (3 × × 10mL). The organic phases were dried over anhydrous MgSO4 and the crude product was obtained after removing the ethereal solution by a rotary evaporator. The product was finally purified by column chromatography on silica gel using petroleum ether/ethyl acetate (10:2) as the solvent or recrystallized.
References:
Dindarloo Inaloo, Iman;Eslahi, Hassan;Esmaeilpour, Mohsen;Majnooni, Sahar [Molecular catalysis,2020,vol. 492]