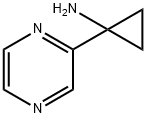
1-(Pyrazin-2-yl)cyclopropan-1-amine synthesis
- Product Name:1-(Pyrazin-2-yl)cyclopropan-1-amine
- CAS Number:1159734-56-1
- Molecular formula:C7H9N3
- Molecular Weight:135.17
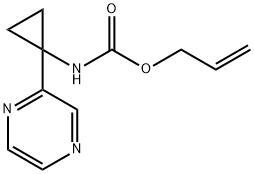
1159734-54-9
1 suppliers
inquiry

1159734-56-1
17 suppliers
inquiry
Yield:1159734-56-1 35%
Reaction Conditions:
with morpholine;tetrakis(triphenylphosphine) palladium(0) in tetrahydrofuran at 50; for 3 h;Inert atmosphere;
Steps:
5.4 Step 4:
To a stirred degassed solution of prop-2-en- 1 -yl [1 -(pyrazin-2-yl)cyclopropyl]carbamate(22, 230 mg, 1.045 mmol) and morpholine (0.9 mL, 10.45 mmol) in THF (10 mL) wasadded Pd(Ph3)4 (72 mg, 0.0627 mmol) and the resulting yellow reaction mixture was stirred at 50°C for 3 hours. After completion (monitored by TLC, 50% EtOAc in hexanes, Rf 0.5), the reaction mixture was concentrated under reduced pressure and the crude residue was purified by column chromatography (100-200 mesh silica, gradient elution of 100% DCM to 5% MeOH in DCM) followed by preparative TLC (TLC Silica gel 60 F254, 20 x 20 cm plates; mobile phase: 1.5% MeOH in DCM) to afford 1-(pyrazin-2- yl)cyclopropanamine (23) as light yellow liquid. Yield: 50mg (35%). 1H NMR (400 MHz,MeOH-d) O 8.82 (d, 1H), 8.46 (d, 1H), 8.33 (d, 1H), 1.31-1.37 (m, 2H), 1.12-1.17 (q,2H). LCMS [M+H]: 136.0
References:
WO2016/193844,2016,A1 Location in patent:Page/Page column 107; 108

1159734-50-5
18 suppliers
inquiry

1159734-56-1
17 suppliers
inquiry
1159734-52-7
37 suppliers
$60.00/1g

1159734-56-1
17 suppliers
inquiry